
Hopanoids are a diverse subclass of triterpenoids with the same hydrocarbon skeleton as the compound hopane. This group of pentacyclic molecules therefore refers to simple hopenes, hopanols and hopanes, but also to extensively functionalized derivatives such as bacteriohopanepolyols (BHPs) and hopanoids covalently attached to lipid A.
Lanosterol is a tetracyclic triterpenoid and is the compound from which all animal and fungal steroids are derived. By contrast, plant steroids are produced via cycloartenol.
(S)-2,3-Oxidosqualene ((S)-2,3-epoxysqualene) is an intermediate in the synthesis of the cell membrane sterol precursors lanosterol and cycloartenol, as well as saponins. It is formed when squalene is oxidized by the enzyme squalene monooxygenase. 2,3-Oxidosqualene is the substrate of various oxidosqualene cyclases, including lanosterol synthase, which produces lanosterol, a precursor to cholesterol.

Lanosterol synthase (EC 5.4.99.7) is an oxidosqualene cyclase (OSC) enzyme that converts (S)-2,3-oxidosqualene to a protosterol cation and finally to lanosterol. Lanosterol is a key four-ringed intermediate in cholesterol biosynthesis. In humans, lanosterol synthase is encoded by the LSS gene.

In enzymology, bornyl diphosphate synthase (BPPS) (EC 5.5.1.8) is an enzyme that catalyzes the chemical reaction
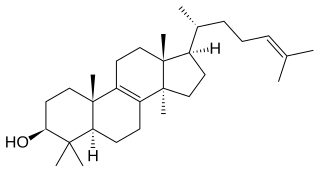
In enzymology, a cycloartenol synthase (EC 5.4.99.8) is an enzyme that catalyzes the chemical reaction
The enzyme aristolochene synthase catalyzes the chemical reaction
The enzyme sabinene-hydrate synthase (EC 4.2.3.11) catalyzes the chemical reaction
Vetispiradiene synthase is an enzyme from Egyptian henbane that catalyzes the following chemical reaction:
In enzymology, a di-trans,poly-cis-decaprenylcistransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a geranyltranstransferase is an enzyme that catalyzes the chemical reaction

Decaprenyl-diphosphate synthase subunit 1 is an enzyme that in humans is encoded by the PDSS1 gene.
Phytoene synthase is a transferase enzyme involved in the biosynthesis of carotenoids. It catalyzes the conversion of geranylgeranyl pyrophosphate to phytoene. This enzyme catalyses the following chemical reaction
The squalene/phytoene synthase family represents proteins that catalyze the head-to-head condensation of C15 and C20 prenyl units (i.e. farnesyl diphosphate and genranylgeranyl diphosphate). This enzymatic step constitutes part of steroid and carotenoid biosynthesis pathway. Squalene synthase EC (SQS) and Phytoene synthase EC (PSY) are two well-known examples of this protein family and share a number of functional similarities. These similarities are also reflected in their primary structure. In particular three well conserved regions are shared by SQS and PSY; they could be involved in substrate binding and/or the catalytic mechanism. SQS catalyzes the conversion of two molecules of farnesyl diphosphate (FPP) into squalene. It is the first committed step in the cholesterol biosynthetic pathway. The reaction carried out by SQS is catalyzed in two separate steps: the first is a head-to-head condensation of the two molecules of FPP to form presqualene diphosphate; this intermediate is then rearranged in a NADP-dependent reduction, to form squalene:
Dammarenediol II synthase (EC 4.2.1.125, dammarenediol synthase, 2,3-oxidosqualene (20S)-dammarenediol cyclase, DDS, (S)-squalene-2,3-epoxide hydro-lyase (dammarenediol-II forming)) is an enzyme with systematic name (3S)-2,3-epoxy-2,3-dihydrosqualene hydro-lyase (dammarenediol-II forming). This enzyme catalyses the following chemical reaction

Squalene-hopene cyclase (SHC) (EC 5.4.99.17) or hopan-22-ol hydro-lyase is an enzyme in the terpene cyclase/mutase family. It catalyzes the interconversion of squalene into a pentacyclic triterpenes, hopene and hopanol. This enzyme catalyses the following chemical reactions.
β-amyrin synthase is an enzyme with systematic name (3S)-2,3-epoxy-2,3-dihydrosqualene mutase . This enzyme catalyses the following chemical reaction
Friedelin synthase is an enzyme with systematic name (3S)-2,3-epoxy-2,3-dihydrosqualene mutase . This enzyme catalyses the following chemical reaction

Oxidosqualene cyclases (OSC) are enzymes involved in cyclization reactions of 2,3-oxidosqualene to form sterols or triterpenes.







