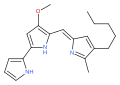
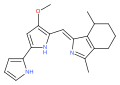
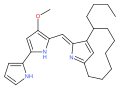
The prodiginines are a family of red tripyrrole dyestuffs produced by Gammaproteobacteria (e.g. Serratia marcescens ) as well as some Actinomycetota (e.g. Streptomyces coelicolor ). The group is named after prodigiosin (prodiginine) and is biosynthesized through a common set of enzymes. [1] They are interesting due to their history and their varied biological activity. [2]