
Neutron activation analysis (NAA) is the nuclear process used for determining the concentrations of elements in a vast amount of materials. NAA allows discrete sampling of elements as it disregards the chemical form of a sample, and focuses solely on its nucleus. The method is based on neutron activation and therefore requires a source of neutrons. The sample is bombarded with neutrons, causing the elements to form radioactive isotopes. The radioactive emissions and radioactive decay paths for each element are well known. Using this information, it is possible to study spectra of the emissions of the radioactive sample, and determine the concentrations of the elements within it. A particular advantage of this technique is that it does not destroy the sample, and thus has been used for analysis of works of art and historical artifacts. NAA can also be used to determine the activity of a radioactive sample.

Spectroscopy is the general field of study that measures and interprets the electromagnetic spectra that result from the interaction between electromagnetic radiation and matter as a function of the wavelength or frequency of the radiation. Matter waves and acoustic waves can also be considered forms of radiative energy, and recently gravitational waves have been associated with a spectral signature in the context of the Laser Interferometer Gravitational-Wave Observatory (LIGO)

Inductively coupled plasma mass spectrometry (ICP-MS) is a type of mass spectrometry that uses an inductively coupled plasma to ionize the sample. It atomizes the sample and creates atomic and small polyatomic ions, which are then detected. It is known and used for its ability to detect metals and several non-metals in liquid samples at very low concentrations. It can detect different isotopes of the same element, which makes it a versatile tool in isotopic labeling.

X-ray photoelectron spectroscopy (XPS) is a surface-sensitive quantitative spectroscopic technique based on the photoelectric effect that can identify the elements that exist within a material or are covering its surface, as well as their chemical state, and the overall electronic structure and density of the electronic states in the material. XPS is a powerful measurement technique because it not only shows what elements are present, but also what other elements they are bonded to. The technique can be used in line profiling of the elemental composition across the surface, or in depth profiling when paired with ion-beam etching. It is often applied to study chemical processes in the materials in their as-received state or after cleavage, scraping, exposure to heat, reactive gasses or solutions, ultraviolet light, or during ion implantation.

X-ray fluorescence (XRF) is the emission of characteristic "secondary" X-rays from a material that has been excited by being bombarded with high-energy X-rays or gamma rays. The phenomenon is widely used for elemental analysis and chemical analysis, particularly in the investigation of metals, glass, ceramics and building materials, and for research in geochemistry, forensic science, archaeology and art objects such as paintings.

Neutron radiation is a form of ionizing radiation that presents as free neutrons. Typical phenomena are nuclear fission or nuclear fusion causing the release of free neutrons, which then react with nuclei of other atoms to form new isotopes—which, in turn, may trigger further neutron radiation. Free neutrons are unstable, decaying into a proton, an electron, plus an electron antineutrino with a mean lifetime of 887 seconds.
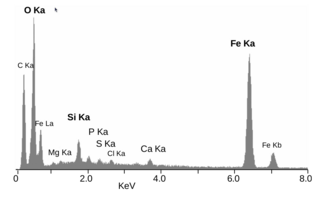
Energy-dispersive X-ray spectroscopy, sometimes called energy dispersive X-ray analysis or energy dispersive X-ray microanalysis (EDXMA), is an analytical technique used for the elemental analysis or chemical characterization of a sample. It relies on an interaction of some source of X-ray excitation and a sample. Its characterization capabilities are due in large part to the fundamental principle that each element has a unique atomic structure allowing a unique set of peaks on its electromagnetic emission spectrum. The peak positions are predicted by the Moseley's law with accuracy much better than experimental resolution of a typical EDX instrument.

The British School at Athens (BSA) is an archaeological research institute, one of the eight British International Research Institutes supported by the British Academy. Under UK law it is a registered educational charity, which translates to a non-profit organisation in American and Greek law. It also is one of the 19 Foreign Archaeological Institutes defined by Hellenic Law No. 3028/2002, "On the Protection of Antiquities and Cultural Heritage in General," passed by the Greek Parliament in 2002. Under that law the 17 accredited foreign institutes may perform systematic excavation in Greece with the permission of the government.

A gamma-ray spectrometer (GRS) is an instrument for measuring the distribution of the intensity of gamma radiation versus the energy of each photon. The study and analysis of gamma-ray spectra for scientific and technical use is called gamma spectroscopy, and gamma-ray spectrometers are the instruments which observe and collect such data. Because the energy of each photon of EM radiation is proportional to its frequency, gamma rays have sufficient energy that they are typically observed by counting individual photons.

Elemental analysis is a process where a sample of some material is analyzed for its elemental and sometimes isotopic composition. Elemental analysis can be qualitative, and it can be quantitative. Elemental analysis falls within the ambit of analytical chemistry, the set of instruments involved in deciphering the chemical nature of our world.

An electron microprobe (EMP), also known as an electron probe microanalyzer (EPMA) or electron micro probe analyzer (EMPA), is an analytical tool used to non-destructively determine the chemical composition of small volumes of solid materials. It works similarly to a scanning electron microscope: the sample is bombarded with an electron beam, emitting x-rays at wavelengths characteristic to the elements being analyzed. This enables the abundances of elements present within small sample volumes to be determined, when a conventional accelerating voltage of 15-20 kV is used. The concentrations of elements from lithium to plutonium may be measured at levels as low as 100 parts per million (ppm), material dependent, although with care, levels below 10 ppm are possible. The ability to quantify lithium by EPMA became a reality in 2008.
Gamma-ray spectroscopy is the quantitative study of the energy spectra of gamma-ray sources, such as in the nuclear industry, geochemical investigation, and astrophysics.

Neutron activation is the process in which neutron radiation induces radioactivity in materials, and occurs when atomic nuclei capture free neutrons, becoming heavier and entering excited states. The excited nucleus decays immediately by emitting gamma rays, or particles such as beta particles, alpha particles, fission products, and neutrons. Thus, the process of neutron capture, even after any intermediate decay, often results in the formation of an unstable activation product. Such radioactive nuclei can exhibit half-lives ranging from small fractions of a second to many years.

Neutron capture is a nuclear reaction in which an atomic nucleus and one or more neutrons collide and merge to form a heavier nucleus. Since neutrons have no electric charge, they can enter a nucleus more easily than positively charged protons, which are repelled electrostatically.

The High Flux Isotope Reactor (HFIR) is a nuclear research reactor at Oak Ridge National Laboratory (ORNL) in Oak Ridge, Tennessee, United States. Operating at 85 MW, HFIR is one of the highest flux reactor-based sources of neutrons for condensed matter physics research in the United States, and it has one of the highest steady-state neutron fluxes of any research reactor in the world. The thermal and cold neutrons produced by HFIR are used to study physics, chemistry, materials science, engineering, and biology. The intense neutron flux, constant power density, and constant-length fuel cycles are used by more than 500 researchers each year for neutron scattering research into the fundamental properties of condensed matter. HFIR has about 600 users each year for both scattering and in-core research.

Coal analyzers are bulk material analyzers used by coal producers, coal preparation plants, and coal-fired power plants to determine coal quality in real time.

The Washington State University Reactor (WSUR) is housed in the Dodgen Research Facility, and was completed in 1961. The (then) Washington State College Reactor was the brainchild of Harold W. Dodgen, a former researcher on the Manhattan Project where he earned his PhD from 1943 to 1946. He secured funding for the ambitious 'Reactor Project' from the National Science Foundation, the Atomic Energy Commission, and the College administration totaling $479,000. Dodgen's basis for constructing a reactor was that the College was primely located as a training facility for the Hanford site, as well as Idaho National Laboratory because there was no other research reactor in the West at that time. After completing the extensive application and design process with the help of contractors from General Electric they broke ground in August 1957 and the first criticality was achieved on March 7, 1961 at a power level of 1W. They gradually increased power over the next year to achieve their maximum licensed operating power of 100 kW.
The term bulk material analyzer is the generic noun for that device which fits around a conveyor belt and conducts real-time elemental analysis of the material on the belt. Other names often found for such a device include belt analyzer, crossbelt analyzer and elemental analyzer. This product first found popularity in the cement industry during the 1990s, and today most new cement plants include at least one analyzer, if not two.
Nuclear forensics is the investigation of nuclear materials to find evidence for the source, the trafficking, and the enrichment of the material. The material can be recovered from various sources including dust from the vicinity of a nuclear facility, or from the radioactive debris following a nuclear explosion.

A radionuclide identification device is a small, lightweight, portable gamma-ray spectrometer used for the detection and identification of radioactive substances. It is available from many companies in various forms to provide hand-held gamma-ray radionuclide identification. Since these instruments are easily carried, they are suitable for first-line responders in key applications of Homeland Security, Environmental Monitoring and Radiological Mapping. These devices have also found their usefulness in medical and industrial applications as well as a number of unique applications such as geological surveys. In the past two decades RIIDs have addressed the growing demand for fast, accurate isotope identification. These light-weight instruments require room temperature detectors so they can be easily carried and perform meaningful measurements in various environments and locations.















