
Propionic acid is a naturally occurring carboxylic acid with chemical formula CH
3CH
2CO
2H. It is a liquid with a pungent and unpleasant smell somewhat resembling body odor. The anion CH
3CH
2CO−
2 as well as the salts and esters of propionic acid are known as propionates or propanoates.

In organic chemistry, thioesters are organosulfur compounds with the molecular structure R−C(=O)−S−R’. They are analogous to carboxylate esters with the sulfur in the thioester replacing oxygen in the carboxylate ester, as implied by the thio- prefix. They are the product of esterification of a carboxylic acid with a thiol. In biochemistry, the best-known thioesters are derivatives of coenzyme A, e.g., acetyl-CoA. The R and R' represent organyl groups, or H in the case of R.
The Friedel–Crafts reactions are a set of reactions developed by Charles Friedel and James Crafts in 1877 to attach substituents to an aromatic ring. Friedel–Crafts reactions are of two main types: alkylation reactions and acylation reactions. Both proceed by electrophilic aromatic substitution.
In organic chemistry, an acyl chloride is an organic compound with the functional group −C(=O)Cl. Their formula is usually written R−COCl, where R is a side chain. They are reactive derivatives of carboxylic acids. A specific example of an acyl chloride is acetyl chloride, CH3COCl. Acyl chlorides are the most important subset of acyl halides.

In organic chemistry, an acyl halide is a chemical compound derived from an oxoacid by replacing a hydroxyl group with a halide group.

Oxalyl chloride is an organic chemical compound with the formula Cl−C(=O)−C(=O)−Cl. This colorless, sharp-smelling liquid, the diacyl chloride of oxalic acid, is a useful reagent in organic synthesis.

Thionyl chloride is an inorganic compound with the chemical formula SOCl2. It is a moderately volatile, colourless liquid with an unpleasant acrid odour. Thionyl chloride is primarily used as a chlorinating reagent, with approximately 45,000 tonnes per year being produced during the early 1990s, but is occasionally also used as a solvent. It is toxic, reacts with water, and is also listed under the Chemical Weapons Convention as it may be used for the production of chemical weapons.
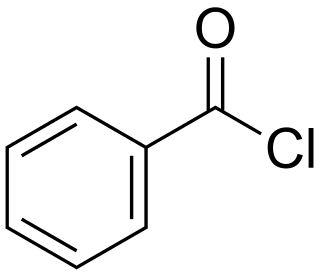
Benzoyl chloride, also known as benzenecarbonyl chloride, is an organochlorine compound with the formula C7H5ClO. It is a colourless, fuming liquid with an irritating odour, and consists of a benzene ring with an acyl chloride substituent. It is mainly useful for the production of peroxides but is generally useful in other areas such as in the preparation of dyes, perfumes, pharmaceuticals, and resins.

In organic chemistry, a sulfoxide, also called a sulphoxide, is an organosulfur compound containing a sulfinyl functional group attached to two carbon atoms. It is a polar functional group. Sulfoxides are oxidized derivatives of sulfides. Examples of important sulfoxides are alliin, a precursor to the compound that gives freshly crushed garlic its aroma, and dimethyl sulfoxide (DMSO), a common solvent.

Cyanuric chloride is an organic compound with the formula (NCCl)3. This white solid is the chlorinated derivative of 1,3,5-triazine. It is the trimer of cyanogen chloride. Cyanuric chloride is the main precursor to the popular but controversial herbicide atrazine.
Nucleophilic acyl substitution describes a class of substitution reactions involving nucleophiles and acyl compounds. In this type of reaction, a nucleophile – such as an alcohol, amine, or enolate – displaces the leaving group of an acyl derivative – such as an acid halide, anhydride, or ester. The resulting product is a carbonyl-containing compound in which the nucleophile has taken the place of the leaving group present in the original acyl derivative. Because acyl derivatives react with a wide variety of nucleophiles, and because the product can depend on the particular type of acyl derivative and nucleophile involved, nucleophilic acyl substitution reactions can be used to synthesize a variety of different products.

In stereochemistry, a chiral auxiliary is a stereogenic group or unit that is temporarily incorporated into an organic compound in order to control the stereochemical outcome of the synthesis. The chirality present in the auxiliary can bias the stereoselectivity of one or more subsequent reactions. The auxiliary can then be typically recovered for future use.
Propionyl-CoA is a coenzyme A derivative of propionic acid. It is composed of a 24 total carbon chain and its production and metabolic fate depend on which organism it is present in. Several different pathways can lead to its production, such as through the catabolism of specific amino acids or the oxidation of odd-chain fatty acids. It later can be broken down by propionyl-CoA carboxylase or through the methylcitrate cycle. In different organisms, however, propionyl-CoA can be sequestered into controlled regions, to alleviate its potential toxicity through accumulation. Genetic deficiencies regarding the production and breakdown of propionyl-CoA also have great clinical and human significance.

2-Chloropropionic acid (2-chloropropanoic acid) is the chemical compound with the formula CH3CHClCO2H. This colorless liquid is the simplest chiral chlorocarboxylic acid, and it is noteworthy for being readily available as a single enantiomer. The conjugate base of 2-chloropropionic acid (CH3CHClCO2−), as well as its salts and esters, are known as 2-chloropropionates or 2-chloropropanoates.

Propionic anhydride is an organic compound with the formula (CH3CH2CO)2O. This simple acid anhydride is a colourless liquid. It is a widely used reagent in organic synthesis as well as for producing specialty derivatives of cellulose.

Chloroacetyl chloride is a chlorinated acyl chloride. It is a bifunctional compound, making it a useful building block chemical.
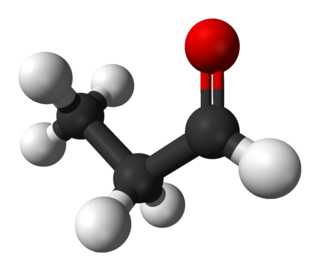
Propionaldehyde or propanal is the organic compound with the formula CH3CH2CHO. It is the 3-carbon aldehyde. It is a colourless, flammable liquid with a pungent and fruity odour. It is produced on a large scale industrially.

In organic chemistry, the sulfonamide functional group is an organosulfur group with the structure R−S(=O)2−NR2. It consists of a sulfonyl group connected to an amine group. Relatively speaking this group is unreactive. Because of the rigidity of the functional group, sulfonamides are typically crystalline; for this reason, the formation of a sulfonamide is a classic method to convert an amine into a crystalline derivative which can be identified by its melting point. Many important drugs contain the sulfonamide group.
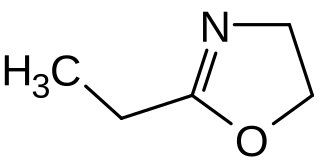
2-Ethyl-2-oxazoline (EtOx) is an oxazoline which is used particularly as a monomer for the cationic ring-opening polymerization to poly(2-alkyloxazoline)s. This type of polymers are under investigation as readily water-soluble and biocompatible materials for biomedical applications.
α-Halo carboxylic acids and esters are organic compounds with the respective formulas RCHXCO2H and RCHXCO2R' where R and R' are organic substituents. The X in these compounds is a halide, usually chloride and bromide. These compounds are often used as intermediates in the preparation of more elaborate derivatives. They are often potent alkylating agents. The mono halide derivatives are chiral.















