Applications



Random packed columns are used in a variety of applications, including:
Random column packing is the practice of packing a distillation column with randomly fitting filtration material in order to optimize surface area over which reactants can interact while minimizing the complexity of construction of such columns. Random column packing is an alternative to structured column packing.
Packed columns utilizing filter media for chemical exchange are the most common devices used in the chemical industry for reactant contact optimization. Packed columns are used in a range of industries to allow intimate contact between two immiscible/partly immiscible fluids, which can be liquid/gas or liquid/liquid. The fluids are passed through a column in a countercurrent flow. In the column it is important to maintain an effective mass transfer, so it is essential that a packing is selected which will support a large surface area for mass transfer. [1]
Random packing was used as early as 1820. Originally the packing material consisted of glass spheres, however in 1850 they were replaced by a more porous pumice stone and pieces of coke.



Random packed columns are used in a variety of applications, including:
The Raschig ring is a piece of tube, invented circa 1914, [2] that is used in large numbers in a packing column. Raschig rings are usually made of ceramic or metals, and they provide a large surface area within the column, allowing for interaction between liquid and gas vapors.
Lessing rings are a type of random packing similar to the Raschig ring invented in the early 20th century by German-born British chemist Rudolf Lessing (1878-1964) of Mond Nickel Company. [3] Originally wrapped from steel strips according to his 1919 patent, [4] now they are made of ceramic. Lessing rings have partitions insides which increase the surface area and enhance mass transfer efficiency. Lessing rings have a high density and an excellent heat and acid resistance. Lessing rings withstand corrosion and are used in regenerative oxide systems and transfer systems.
Pall rings are the most common form of random packing. They are similar to Lessing rings and were developed from the Raschig ring. Pall rings have similar cylindrical dimensions but has rows of windows which increase performance by increasing the surface area. They are suited for low pressure drop and high capacity applications. They have a degree of randomness and a relatively high liquid hold up, promoting a high absorption, especially when the rate of reaction is slow. The cross structure of the Pall ring makes it mechanically robust and suitable for use in deep packed beds.
The Bialecki ring was patented in 1974 by Polish chemical engineer from Kraków Zbigniew Białecki rings are an improved version of Raschig rings. The rings may be injection moulded of plastics or press-formed from metal sheet without welding. Specific surface area of filling ranges between 60 and 440 m2/m3. [5]
Dixon rings have a similar design to Lessing rings. They are made of stainless steel mesh, giving Dixon rings a low pressure drop and after pre-wetting. Dixon rings have a very large surface area, which increases the rate of mass transfer. Dixon rings have a large liquid hold up, a low pressure drop and a large surface area, and have a high mass transfer rate. Dixon rings are used for laboratory distillation and scrubbing applications.

Distillation, also classical distillation, is the process of separating the component substances of a liquid mixture of two or more chemically discrete substances; the separation process is realized by way of the selective boiling of the mixture and the condensation of the vapors in a still.
Fractional distillation is the separation of a mixture into its component parts, or fractions. Chemical compounds are separated by heating them to a temperature at which one or more fractions of the mixture will vaporize. It uses distillation to fractionate. Generally the component parts have boiling points that differ by less than 25 °C (45 °F) from each other under a pressure of one atmosphere. If the difference in boiling points is greater than 25 °C, a simple distillation is typically used.

A Raschig ring is a piece of tube, approximately equal in length and diameter, used in large numbers as a packed bed within columns for distillations and other chemical engineering processes. They are usually ceramic, metal, or glass and provide a large surface area within the volume of the column for interaction between liquid and gas vapours.

A fractionating column or fractional column is equipment used in the distillation of liquid mixtures to separate the mixture into its component parts, or fractions, based on their differences in volatility. Fractionating columns are used in small-scale laboratory distillations as well as large-scale industrial distillations.
Chemical kinetics, also known as reaction kinetics, is the branch of physical chemistry that is concerned with understanding the rates of chemical reactions. It is different from chemical thermodynamics, which deals with the direction in which a reaction occurs but in itself tells nothing about its rate. Chemical kinetics includes investigations of how experimental conditions influence the speed of a chemical reaction and yield information about the reaction's mechanism and transition states, as well as the construction of mathematical models that also can describe the characteristics of a chemical reaction.

Vacuum distillation or distillation under reduced pressure is a type of distillation performed under reduced pressure, which allows the purification of compounds not readily distilled at ambient pressures or simply to save time or energy. This technique separates compounds based on differences in their boiling points. This technique is used when the boiling point of the desired compound is difficult to achieve or will cause the compound to decompose. Reduced pressures decrease the boiling point of compounds. The reduction in boiling point can be calculated using a temperature-pressure nomograph using the Clausius–Clapeyron relation.

Continuous distillation, a form of distillation, is an ongoing separation in which a mixture is continuously fed into the process and separated fractions are removed continuously as output streams. Distillation is the separation or partial separation of a liquid feed mixture into components or fractions by selective boiling and condensation. The process produces at least two output fractions. These fractions include at least one volatile distillate fraction, which has boiled and been separately captured as a vapor condensed to a liquid, and practically always a bottoms fraction, which is the least volatile residue that has not been separately captured as a condensed vapor.

In chemical processing, a packed bed is a hollow tube, pipe, or other vessel that is filled with a packing material. The packed bed can be randomly filled with small objects like Raschig rings or else it can be a specifically designed structured packing. Packed beds may also contain catalyst particles or adsorbents such as zeolite pellets, granular activated carbon, etc.

The term structured packing refers to a range of specially designed materials for use in absorption and distillation columns and chemical reactors. Structured packings typically consist of thin corrugated metal plates or gauzes arranged in a way that force fluids to take complicated paths through the column, thereby creating a large surface area for contact between different phases.
A theoretical plate in many separation processes is a hypothetical zone or stage in which two phases, such as the liquid and vapor phases of a substance, establish an equilibrium with each other. Such equilibrium stages may also be referred to as an equilibrium stage, ideal stage, or a theoretical tray. The performance of many separation processes depends on having series of equilibrium stages and is enhanced by providing more such stages. In other words, having more theoretical plates increases the efficiency of the separation process be it either a distillation, absorption, chromatographic, adsorption or similar process.
The term wet scrubber describes a variety of devices that remove pollutants from a furnace flue gas or from other gas streams. In a wet scrubber, the polluted gas stream is brought into contact with the scrubbing liquid, by spraying it with the liquid, by forcing it through a pool of liquid, or by some other contact method, so as to remove the pollutants.
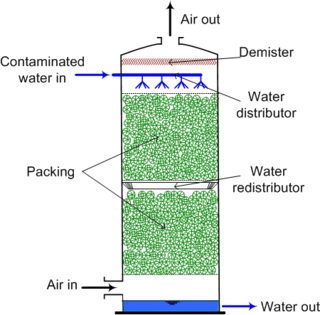
Air stripping is the transferring of volatile components of a liquid into an air stream. It is an environmental engineering technology used for the purification of groundwaters and wastewaters containing volatile compounds.

In chemistry, a condenser is laboratory apparatus used to condense vapors – that is, turn them into liquids – by cooling them down.
Stripping is a physical separation process where one or more components are removed from a liquid stream by a vapor stream. In industrial applications the liquid and vapor streams can have co-current or countercurrent flows. Stripping is usually carried out in either a packed or trayed column.
A monolithic HPLC column, or monolithic column, is a column used in high-performance liquid chromatography (HPLC). The internal structure of the monolithic column is created in such a way that many channels form inside the column. The material inside the column which separates the channels can be porous and functionalized. In contrast, most HPLC configurations use particulate packed columns; in these configurations, tiny beads of an inert substance, typically a modified silica, are used inside the column. Monolithic columns can be broken down into two categories, silica-based and polymer-based monoliths. Silica-based monoliths are known for their efficiency in separating smaller molecules while, polymer-based are known for separating large protein molecules.
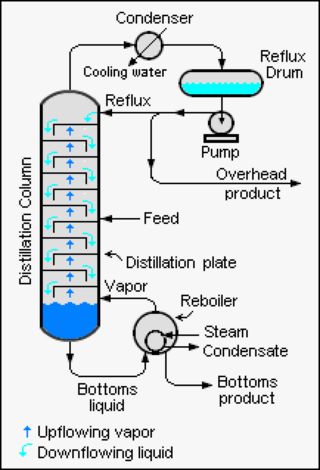
Reflux is a technique involving the condensation of vapors and the return of this condensate to the system from which it originated. It is used in industrial and laboratory distillations. It is also used in chemistry to supply energy to reactions over a long period of time.
Catalytic distillation is a branch of reactive distillation which combines the processes of distillation and catalysis to selectively separate mixtures within solutions. Its main function is to maximize the yield of catalytic organic reactions, such as the refining of gasoline. The earliest case of catalytic distillation was thought to have dated back to 1966; however, the idea was officially patented in 1980 by Lawrence A. Smith, Jr. The process is currently used to purify gasoline, extract rubber, and form plastics.
A gas–liquid contactor is a particular chemical equipment used to realize the mass and heat transfer between a gas phase and a liquid phase. Gas–liquid contactors can be used in separation processes or as gas–liquid reactors or to achieve both purposes within the same device.
Industrial separation processes are technical procedures which are used in industry to separate a product from impurities or other products. The original mixture may either be a natural resource or the product of a chemical reaction.
Dixon rings are a form of random packing used in chemical processing. They consist of a stainless steel mesh formed into a ring with a central divider, and are intended to be packed randomly into a packed column. Dixon rings provide a large surface area and low pressure drop while maintaining a high mass transfer rate, making them useful for distillations and many other applications.