
β-Carboline (9H-pyrido[3,4-b]indole) represents the basic chemical structure for more than one hundred alkaloids and synthetic compounds. The effects of these substances depend on their respective substituent. Natural β-carbolines primarily influence brain functions but can also exhibit antioxidant effects. Synthetically designed β-carboline derivatives have recently been shown to have neuroprotective, cognitive enhancing and anti-cancer properties.
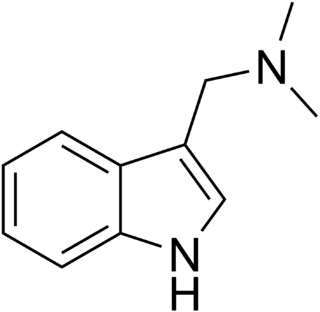
Gramine is a naturally occurring indole alkaloid present in several plant species. Gramine may play a defensive role in these plants, since it is toxic to many organisms.

Rauvolfia serpentina, the Indian snakeroot, devil pepper, or serpentine wood, is a species of flower in the milkweed family Apocynaceae. It is native to the Indian subcontinent and East Asia.

Voacangine is an alkaloid found predominantly in the root bark of the Voacanga africana tree, as well as in other plants such as Tabernanthe iboga, Tabernaemontana africana, Trachelospermum jasminoides, Tabernaemontana divaricata and Ervatamia yunnanensis. It is an iboga alkaloid which commonly serves as a precursor for the semi-synthesis of ibogaine. It has been demonstrated in animals to have similar anti-addictive properties to ibogaine itself. It also potentiates the effects of barbiturates. Under UV-A and UV-B light its crystals fluoresce blue-green, and it is soluble in ethanol.

Indole alkaloids are a class of alkaloids containing a structural moiety of indole; many indole alkaloids also include isoprene groups and are thus called terpene indole or secologanin tryptamine alkaloids. Containing more than 4100 known different compounds, it is one of the largest classes of alkaloids. Many of them possess significant physiological activity and some of them are used in medicine. The amino acid tryptophan is the biochemical precursor of indole alkaloids.
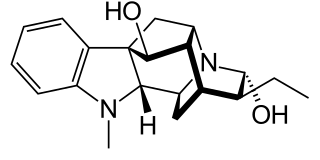
Ajmaline is an alkaloid that is classified as a 1-A antiarrhythmic agent. It is often used to induce arrhythmic contraction in patients suspected of having Brugada syndrome. Individuals suffering from Brugada syndrome will be more susceptible to the arrhythmogenic effects of the drug, and this can be observed on an electrocardiogram as an ST elevation.

7-Hydroxymitragynine (7-OH) is a terpenoid indole alkaloid from the plant Mitragyna speciosa, commonly known as kratom. It was first described in 1994 and is a natural product derived from the mitragynine present in the kratom leaf. 7-OH binds to opioid receptors like mitragynine, but research suggests that 7-OH binds with greater potency.
In enzymology, a 1,2-dihydrovomilenine reductase (EC 1.3.1.73) is an enzyme that catalyzes the chemical reaction
Strictosidine synthase (EC 4.3.3.2) is an enzyme in alkaloid biosynthesis that catalyses the condensation of tryptamine with secologanin to form strictosidine in a formal Pictet–Spengler reaction:
In enzymology, a vinorine synthase is an enzyme that catalyzes the chemical reaction

Ajmalicine, also known as δ-yohimbine or raubasine, is an antihypertensive drug used in the treatment of high blood pressure. It has been marketed under numerous brand names including Card-Lamuran, Circolene, Cristanyl, Duxil, Duxor, Hydroxysarpon, Iskedyl, Isosarpan, Isquebral, Lamuran, Melanex, Raunatin, Saltucin Co, Salvalion, and Sarpan. It is an alkaloid found naturally in various plants such as Rauvolfia spp., Catharanthus roseus, and Mitragyna speciosa.
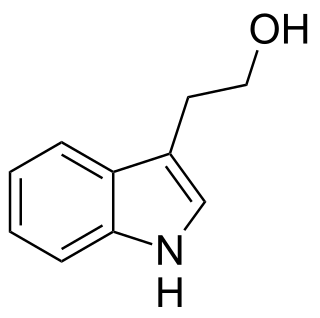
Tryptophol is an aromatic alcohol that induces sleep in humans. It is found in wine as a secondary product of ethanol fermentation. It was first described by Felix Ehrlich in 1912. It is also produced by the trypanosomal parasite in sleeping sickness.
Perakine reductase (EC 1.1.1.317) is an enzyme with systematic name raucaffrinoline:NADP+ oxidoreductase. This enzyme catalyses the following chemical reaction
3α(S)-strictosidine β-glucosidase (EC 3.2.1.105) is an enzyme with systematic name strictosidine β-D-glucohydrolase. It catalyses the following chemical reaction:

Akuammicine is a monoterpene indole alkaloid of the Vinca sub-group. It is found in the Apocynaceae family of plants including Picralima nitida, Vinca minor and the Aspidosperma.

Perakine is an indole alkaloid isolated from the leaves of Rauvolfia yunnanensis.
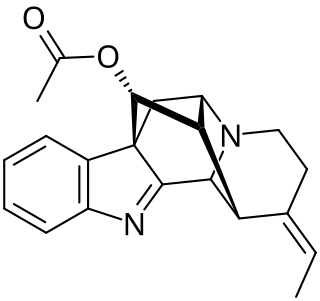
Vinorine is an indole alkaloid isolated from Alstonia.

Rauvolfia nukuhivensis is a species of plant in the family Apocynaceae. It is endemic to Nuku Hiva in the Marquesas Islands in French Polynesia.

Apparicine is a monoterpenoid indole alkaloid. It is named after Apparicio Duarte, a Brazilian botanist who studied the Aspidosperma species from which apparicine was first isolated. It was the first member of the vallesamine group of alkaloids to be isolated and have its structure established, which was first published in 1965. It has also been known by the synonyms gomezine, pericalline, and tabernoschizine.

Conophylline is a autophagy inducing vinca alkaloid found in several species of Tabernaemontana including Ervatamia microphylla and Tabernaemontana divaricata. Among its many functional groups is an epoxide: the compound where that ring is replaced with a double bond is called conophyllidine and this co-occurs in the same plants.















