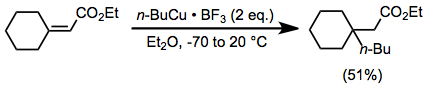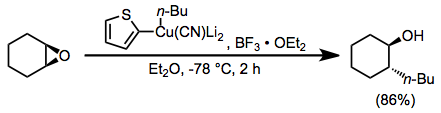A Gilman reagent is a lithium and copper (diorganocopper) reagent compound, R2CuLi, where R is an alkyl or aryl. These reagents are useful because, unlike related Grignard reagents and organolithium reagents, they react with organic halides to replace the halide group with an R group (the Corey–House reaction). Such displacement reactions allow for the synthesis of complex products from simple building blocks.
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively charged, have an atom that carries a partial positive charge, or have an atom that does not have an octet of electrons.

Organolithium reagents are organometallic compounds that contain carbon–lithium bonds. These reagents are important in organic synthesis, and are frequently used to transfer the organic group or the lithium atom to the substrates in synthetic steps, through nucleophilic addition or simple deprotonation. Organolithium reagents are used in industry as an initiator for anionic polymerization, which leads to the production of various elastomers. They have also been applied in asymmetric synthesis in the pharmaceutical industry. Due to the large difference in electronegativity between the carbon atom and the lithium atom, the C−Li bond is highly ionic. Owing to the polar nature of the C−Li bond, organolithium reagents are good nucleophiles and strong bases. For laboratory organic synthesis, many organolithium reagents are commercially available in solution form. These reagents are highly reactive, and are sometimes pyrophoric.

Copper(I) chloride, commonly called cuprous chloride, is the lower chloride of copper, with the formula CuCl. The substance is a white solid sparingly soluble in water, but very soluble in concentrated hydrochloric acid. Impure samples appear green due to the presence of copper(II) chloride (CuCl2).
Metalation is a chemical reaction that forms a bond to a metal. This reaction usually refers to the replacement of a halogen atom in an organic molecule with a metal atom, resulting in an organometallic compound. In the laboratory, metalation is commonly used to activate organic molecules during the formation of C—X bonds, which are necessary for the synthesis of many organic molecules.
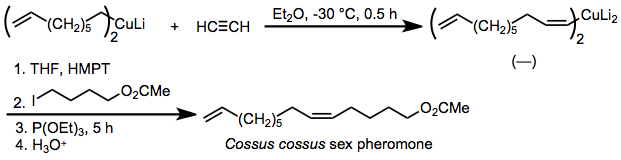
The Corey–House synthesis (also called the Corey–Posner–Whitesides–House reaction and other permutations) is an organic reaction that involves the reaction of a lithium diorganylcuprate (R2CuLi) with an organic pseudohalide (R'X) to form a new alkane, as well as an ill-defined organocopper species and lithium halide as byproducts.
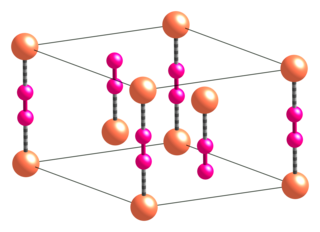
Copper(I) cyanide is an inorganic compound with the formula CuCN. This off-white solid occurs in two polymorphs; impure samples can be green due to the presence of Cu(II) impurities. The compound is useful as a catalyst, in electroplating copper, and as a reagent in the preparation of nitriles.

A Grignard reagent or Grignard compound is a chemical compound with the generic formula R−Mg−X, where X is a halogen and R is an organic group, normally an alkyl or aryl. Two typical examples are methylmagnesium chloride Cl−Mg−CH3 and phenylmagnesium bromide (C6H5)−Mg−Br. They are a subclass of the organomagnesium compounds.

Methyllithium is the simplest organolithium reagent with the empirical formula CH3Li. This s-block organometallic compound adopts an oligomeric structure both in solution and in the solid state. This highly reactive compound, invariably used in solution with an ether as the solvent, is a reagent in organic synthesis as well as organometallic chemistry. Operations involving methyllithium require anhydrous conditions, because the compound is highly reactive toward water. Oxygen and carbon dioxide are also incompatible with MeLi. Methyllithium is usually not prepared, but purchased as a solution in various ethers.

Organozinc compounds in organic chemistry contain carbon to zinc chemical bonds. Organozinc chemistry is the science of organozinc compounds describing their physical properties, synthesis and reactions.

Organocopper compounds in organometallic chemistry contain carbon to copper chemical bonds. Organocopper chemistry is the science of organocopper compounds describing their physical properties, synthesis and reactions. They are reagents in organic chemistry.

A boronic acid is a compound related to boric acid in which one of the three hydroxyl groups is replaced by an alkyl or aryl group. As a compound containing a carbon–boron bond, members of this class thus belong to the larger class of organoboranes. Boronic acids act as Lewis acids. Their unique feature is that they are capable of forming reversible covalent complexes with sugars, amino acids, hydroxamic acids, etc.. The pKa of a boronic acid is ~9, but they can form tetrahedral boronate complexes with pKa ~7. They are occasionally used in the area of molecular recognition to bind to saccharides for fluorescent detection or selective transport of saccharides across membranes.
A carbometalation is any reaction where a carbon-metal bond reacts with a carbon-carbon π-bond to produce a new carbon-carbon σ-bond and a carbon-metal σ-bond. The resulting carbon-metal bond can undergo further carbometallation reactions or it can be reacted with a variety of electrophiles including halogenating reagents, carbonyls, oxygen, and inorganic salts to produce different organometallic reagents. Carbometalations can be performed on alkynes and alkenes to form products with high geometric purity or enantioselectivity, respectively. Some metals prefer to give the anti-addition product with high selectivity and some yield the syn-addition product. The outcome of syn and anti- addition products is determined by the mechanism of the carbometalation.
Vicinal difunctionalization refers to a chemical reaction involving transformations at two adjacent centers. This transformation can be accomplished in α,β-unsaturated carbonyl compounds via the conjugate addition of a nucleophile to the β-position followed by trapping of the resulting enolate with an electrophile at the α-position. When the nucleophile is an enolate and the electrophile a proton, the reaction is called Michael addition.
Electrophilic amination is a chemical process involving the formation of a carbon–nitrogen bond through the reaction of a nucleophilic carbanion with an electrophilic source of nitrogen.
The Payne rearrangement is the isomerization, under basic conditions, of 2,3-epoxy alcohols to isomeric 1,2-epoxy alcohols with inversion of configuration. Aza- and thia-Payne rearrangements of aziridines and thiiraniums, respectively, are also known.
Reactions of alkenyl- and alkynylaluminium compounds involve the transfer of a nucleophilic alkenyl or alkynyl group attached to aluminium to an electrophilic atom. Stereospecific hydroalumination, carboalumination, and terminal alkyne metalation are useful methods for generation of the necessary alkenyl- and alkynylalanes.
Heteroatom-promoted lateral lithiation is the site-selective replacement of a benzylic hydrogen atom for lithium for the purpose of further functionalization. Heteroatom-containing substituents may direct metalation to the benzylic site closest to the heteroatom or increase the acidity of the ring carbons via an inductive effect.
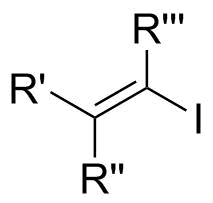
In organic chemistry, a vinyl iodide functional group is an alkene with one or more iodide substituents. Vinyl iodides are versatile molecules that serve as important building blocks and precursors in organic synthesis. They are commonly used in carbon-carbon forming reactions in transition-metal catalyzed cross-coupling reactions, such as Stille reaction, Heck reaction, Sonogashira coupling, and Suzuki coupling. Synthesis of well-defined geometry or complexity vinyl iodide is important in stereoselective synthesis of natural products and drugs.
In organometallic chemistry, metal–halogen exchange is a fundamental reaction that converts a organic halide into an organometallic product. The reaction commonly involves the use of electropositive metals and organochlorides, bromides, and iodides. Particularly well-developed is the use of metal–halogen exchange for the preparation of organolithium compounds.