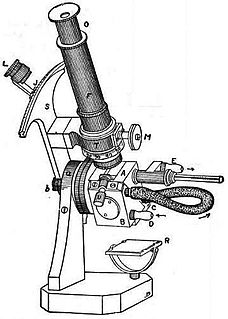
Refractometry is the analytical method of measuring substances' refractive index (one of their fundamental physical properties) in order to, for example, assess their composition or purity. A refractometer is the instrument used to measure refractive index ("RI"). Although refractometers are best known for measuring liquids, they are also used to measure gases and solids; such as glass and gemstones.

In optics, the refractive index or index of refraction of a material is a dimensionless number that describes how fast light travels through the material. It is defined as
A physical property is any property that is measurable, whose value describes a state of a physical system. The changes in the physical properties of a system can be used to describe its changes between momentary states. Physical properties are often referred to as observables. They are not modal properties. Quantifiable physical property is called physical quantity.
A refractometer is a laboratory or field device for the measurement of an index of refraction (refractometry). The index of refraction is calculated from Snell's law while for mixtures, the index of refraction can be calculated from the composition of the material using several mixing rules such as the Gladstone–Dale relation and Lorentz–Lorenz equation.
Contents
The RI of a substance is strongly influenced by temperature and the wavelength of light used to measure it, therefore, care must be taken to control or compensate for temperature differences and wavelength. RI measurements are usually reported at a reference temperature of 20 degrees Celsius, which is equal to 68 degrees Fahrenheit, and considered to be room temperature. A reference wavelength of 589.3 nm (the sodium D line) is most often used. Though RI is a dimensionless quantity, it is typically reported as nD20 (or n20
D ), where the "n" represents refractive index, the "D" denotes the wavelength, and the 20 denotes the reference temperature. Therefore, the refractive index of water at 20 degrees Celsius, taken at the Sodium D Line, would be reported as 1.3330 nD20.
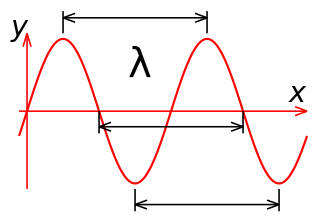
In physics, the wavelength is the spatial period of a periodic wave—the distance over which the wave's shape repeats. It is the distance between consecutive corresponding points of the same phase on the wave, such as two adjacent crests, troughs, or zero crossings, and is a characteristic of both traveling waves and standing waves, as well as other spatial wave patterns. The inverse of the wavelength is called the spatial frequency. Wavelength is commonly designated by the Greek letter lambda (λ). The term wavelength is also sometimes applied to modulated waves, and to the sinusoidal envelopes of modulated waves or waves formed by interference of several sinusoids.

The Celsius scale, also known as the centigrade scale, is a temperature scale used by the International System of Units (SI). As an SI derived unit, it is used worldwide. In the United States, the Bahamas, Belize, the Cayman Islands and Liberia however, Fahrenheit remains the preferred scale for everyday temperature measurement. The degree Celsius can refer to a specific temperature on the Celsius scale or a unit to indicate a difference between two temperatures or an uncertainty. It is named after the Swedish astronomer Anders Celsius (1701–1744), who developed a similar temperature scale. Before being renamed to honor Anders Celsius in 1948, the unit was called centigrade, from the Latin centum, which means 100, and gradus, which means steps.

The Fahrenheit scale is a temperature scale based on one proposed in 1724 by German physicist Daniel Gabriel Fahrenheit (1686–1736). It uses the degree Fahrenheit as the unit. Several accounts of how he originally defined his scale exist. The lower defining point, 0 ℉, was established as the freezing temperature of a solution of brine made from equal parts of ice, water and a salt. Further limits were established as the melting point of ice (32 ℉) and his best estimate of the average human body temperature. The scale is now usually defined by two fixed points: the temperature at which water freezes into ice is defined as 32 ℉, and the boiling point of water is defined to be 212 ℉, a 180 ℉ separation, as defined at sea level and standard atmospheric pressure.
Refractometers are frequently used by grape growers and kiwifruit growers for Brix testing of sucrose levels in their fruit. Refractometry is also used in the gelatin industry. To convert the RI of a gelatin sol (reported in Brix) to a gelatin concentration, one need only multiply by eight-tenths (0.8). A sol with a 10.0 RI would therefore be 8% gelatin by weight. This is known to be a reliable conversion for gelatin sols as low as 1% up to over 50%.

Viticulture or winegrowing is the cultivation and harvesting of grapes. It is a branch of the science of horticulture. While the native territory of Vitis vinifera, the common grape vine, ranges from Western Europe to the Persian shores of the Caspian Sea, the vine has demonstrated high levels of adaptability to new environments; thus, viticulture can be found on every continent except Antarctica.

Kiwifruit, or Chinese gooseberry, is the edible berry of several species of woody vines in the genus Actinidia. The most common cultivar group of kiwifruit is oval, about the size of a large hen's egg. It has a thin, hair-like, fibrous, sour-but-edible light brown skin and light green or golden flesh with rows of tiny, black, edible seeds. The fruit has a soft texture with a sweet and unique flavour. China produced 50% of the world total of kiwifruit in 2017.
Degrees Brix is the sugar content of an aqueous solution. One degree Brix is 1 gram of sucrose in 100 grams of solution and represents the strength of the solution as percentage by mass. If the solution contains dissolved solids other than pure sucrose, then the °Bx only approximates the dissolved solid content. The °Bx is traditionally used in the wine, sugar, carbonated beverage, fruit juice, maple syrup and honey industries.