Regenerative Medicine Advanced Therapy (RMAT) is a designation given by the Food and Drug Administration to drug candidates intended to treat serious or life-threatening conditions under the 21st Century Cures Act. [1] A RMAT designation allows for accelerated approval based surrogate or intermediate endpoints. [2]
RMAT goes beyond breakthrough therapy features by allowing for accelerated approval of drugs based on surrogate endpoints. A surrogate endpoint is a biomarker that substitutes for a direct endpoint, such as clinical benefit. [3]
Section 3033 of the 21st Century Cures Act introduces section 506(g) of the Federal Food, Drug, and Cosmetic Act (FD&C Act) that allows for the designation of certain therapies as a 'regenerative medicine advanced therapy' (RMAT) (21 U.S.C. § 356).
In order to qualify for RMAT status, a treatment must
A regenerative medicine therapy is defined in section 506(g)(8) of the FD&C Act to include cell therapies, therapeutic tissue engineering, human cell and tissue products. Under the FDA's interpretation, gene therapies and genetically modified cells that have a lasting effect, such as CAR-T antitumor therapies, may also qualify as regenerative medicine therapies.
A RMAT designation includes all benefits of the Fast Track and breakthrough therapy designations. In addition, it opens up early interactions between the FDA and sponsors to facilitate accelerated approval. In this context, accelerated approval means approval based on
The ability to use 'Real World Evidence' (RWE), i.e. post-market evidence of safety and effectiveness, is particularly useful in the context of orphan diseases, where recruiting a sufficiently large cohort for pre-marketing clinical trials may not be feasible. [5] RWE may include data from patient registries, clinical records and case studies. [6]
Where a RMAT's sponsor fails to comply with the requirements for accelerated approval, the RMAT designation and the benefits conferred by it can be withdrawn ( 21 CFR 601.43 ).
In 2020, the FDA received 34 requests for RMAT status, of which 12 (35.3%) were granted. RMAT designated drugs include the novel CAR-T therapy Kymriah and betibeglogene autotemcel for beta thalassemia. [33] As of 31 March 2021, 62 requests for RMAT status have been granted. [34]
More than half of the RMAT applications received by March 2019 involved autologous or allogeneic cell therapy products, including CAR-T therapies. [6]
Sarepta Therapeutics, Inc. is a medical research and drug development company with corporate offices and research facilities in Cambridge, Massachusetts, United States. Incorporated in 1980 as AntiVirals, shortly before going public the company changed its name from AntiVirals to AVI BioPharma soon with stock symbol AVII and in July 2012 changed name from AVI BioPharma to Sarepta Therapeutics and SRPT respectively. As of 2023, the company has four approved drugs.

Fast track is a designation by the United States Food and Drug Administration (FDA) of an investigational drug for expedited review to facilitate development of drugs that treat a serious or life-threatening condition and fill an unmet medical need. Fast track designation must be requested by the drug company. The request can be initiated at any time during the drug development process. FDA will review the request and attempt to make a decision within sixty days.
Astellas Institute for Regenerative Medicine is a subsidiary of Astellas Pharma located in Marlborough, Massachusetts, US, developing stem cell therapies with a focus on diseases that cause blindness. It was formed in 1994 as a company named Advanced Cell Technology, Incorporated (ACT), which was renamed to Ocata Therapeutics in November 2014. In February 2016 Ocata was acquired by Astellas for $379 million USD.
Teprotumumab, sold under the brand name Tepezza, is a medication used to treat adults with thyroid eye disease, a rare condition where the muscles and fatty tissues behind the eye become inflamed, causing the eyes to bulge outwards.
Flexion Therapeutics is an American biopharmaceutical company based in Burlington, Massachusetts that is focused on the discovery, development and commercialization of novel, local therapies for the treatment of patients with musculoskeletal conditions, beginning with osteoarthritis (OA), the most common form of arthritis.
Arcturus Therapeutics is an American RNA medicines biotechnology company focused on the discovery, development and commercialization of therapeutics for rare diseases and infectious diseases. Arcturus has developed proprietary lipid nanoparticle RNA therapeutics for nucleic acid medicines including small interfering RNA (siRNA), messenger RNA (mRNA), gene editing RNA, DNA, antisense oligonucleotides, and microRNA.
Adverum Biotechnologies, formerly known as Avalanche Biotechnologies, is a publicly traded clinical stage gene therapy company located in Redwood City, California. The company is targeting unmet medical needs for serious ocular and rare diseases, including wet age-related macular degeneration.
Inebilizumab, sold under the brand name Uplizna, is a medication for the treatment of neuromyelitis optica spectrum disorder (NMOSD) in adults. Inebilizumab is a humanized mAb that binds to and depletes CD19+ B cells including plasmablasts and plasma cells.
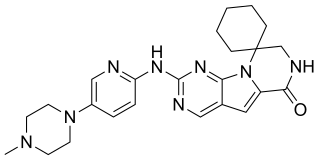
Trilaciclib, sold under the brand name Cosela, is a medication used to reduce the frequency of chemotherapy-induced bone marrow suppression.
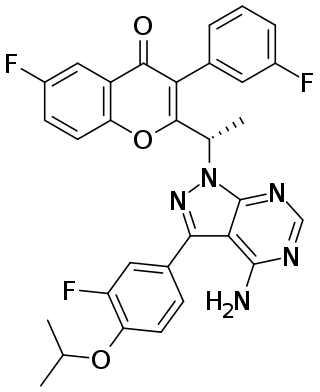
Umbralisib, sold under the brand name Ukoniq, is an anti-cancer medication for the treatment of marginal zone lymphoma (MZL) and follicular lymphoma (FL). It is taken by mouth.
Lisocabtagene maraleucel, sold under the brand name Breyanzi, is a cell-based gene therapy used to treat large B-cell lymphoma.
bluebird bio, Inc., based in Somerville, Massachusetts, is a biotechnology company that develops gene therapies for severe genetic disorders.

Pemigatinib, sold under the brand name Pemazyre, is an anti-cancer medication used for the treatment of bile duct cancer (cholangiocarcinoma). Pemigatinib works by blocking FGFR2 in tumor cells to prevent them from growing and spreading.

Ultragenyx is an American biopharmaceutical company involved in the research and development of novel products for treatment of rare and ultra-rare genetic diseases for which there are typically no approved treatments and high unmet medical need. The company works with multiple drug modalities including biologics, small molecule, gene therapies, and ASO and mRNAs in the disease categories of bone, endocrine, metabolic, muscle and CNS diseases.

CRISPR Therapeutics AG is a Swiss–American biotechnology company headquartered in Zug, Switzerland. It was one of the first companies formed to utilize the CRISPR gene editing platform to develop medicines for the treatment of various rare and common diseases. The company has approximately 500 employees and has offices in Zug, Switzerland, Boston, Massachusetts, San Francisco, California and London, United Kingdom. Its manufacturing facility in Framingham, Massachusetts won the Facilities of the Year Award (FOYA) award in 2022. The company’s lead program, exagamglogene autotemcel, or exa-cel, was granted regulatory approval in December 2023.
Infigratinib, sold under the brand name Truseltiq, is an anti-cancer medication used to treat cholangiocarcinoma.
OTL-103 (GSK-2696275) is a gene therapy for Wiskott–Aldrich syndrome, a rare primary immunodeficiency caused by mutations in the gene that codes for Wiskott–Aldrich syndrome protein (WASp). It was developed by Orchard Therapeutics in conjunction with GlaxoSmithKline. It is currently undergoing Phase I/II of clinical trials that are expected to conclude in October 2025.
RVT-802 is a medication being developed by Enzyvant Therapeutics Ireland Limited for the treatment of congenital athymia, especially in the context of DiGeorge syndrome.
Valoctocogene roxaparvovec, sold under the brand name Roctavian, is a gene therapy used for the treatment of hemophilia A. It was developed by BioMarin Pharmaceutical. Valoctocogene roxaparvovec is made of a virus (AAV5) that has been modified to contain the gene for factor VIII, which is lacking in people with hemophilia A. It is an adeno-associated virus vector-based gene therapy. It is given by intravenous infusion.
Precision BioSciences, Inc. is a publicly traded American clinical stage gene editing company headquartered in Durham, North Carolina. Founded in 2006, Precision is focused on developing both in vivo and ex vivo gene editing therapies using its proprietary "ARCUS" genome editing platform.
{{cite web}}: Check |url= value (help)[ permanent dead link ]{{cite web}}: Check |url= value (help)[ permanent dead link ]