Related Research Articles
An electrolyte is a medium containing ions that is electrically conducting through the movement of those ions, but not conducting electrons. This includes most soluble salts, acids, and bases dissolved in a polar solvent, such as water. Upon dissolving, the substance separates into cations and anions, which disperse uniformly throughout the solvent. Solid-state electrolytes also exist. In medicine and sometimes in chemistry, the term electrolyte refers to the substance that is dissolved.
The chloride ion is the anion Cl−. It is formed when the element chlorine gains an electron or when a compound such as hydrogen chloride is dissolved in water or other polar solvents. Chloride salts such as sodium chloride are often very soluble in water. It is an essential electrolyte located in all body fluids responsible for maintaining acid/base balance, transmitting nerve impulses and regulating liquid flow in and out of cells. Less frequently, the word chloride may also form part of the "common" name of chemical compounds in which one or more chlorine atoms are covalently bonded. For example, methyl chloride, with the standard name chloromethane is an organic compound with a covalent C−Cl bond in which the chlorine is not an anion.

Laxatives, purgatives, or aperients are substances that loosen stools and increase bowel movements. They are used to treat and prevent constipation.

Ascites is the abnormal build-up of fluid in the abdomen. Technically, it is more than 25 ml of fluid in the peritoneal cavity, although volumes greater than one liter may occur. Symptoms may include increased abdominal size, increased weight, abdominal discomfort, and shortness of breath. Complications can include spontaneous bacterial peritonitis.

Sports drinks, also known as electrolyte drinks, are functional beverages whose stated purpose is to help athletes replace water, electrolytes, and energy before, during and especially after training or competition. There are many perceived benefits and questions pertaining to the efficacy of use for sports and fitness performance.

Electrolyte imbalance, or water-electrolyte imbalance, is an abnormality in the concentration of electrolytes in the body. Electrolytes play a vital role in maintaining homeostasis in the body. They help to regulate heart and neurological function, fluid balance, oxygen delivery, acid–base balance and much more. Electrolyte imbalances can develop by consuming too little or too much electrolyte as well as excreting too little or too much electrolyte.
Hyperchloremia is an electrolyte disturbance in which there is an elevated level of chloride ions in the blood. The normal serum range for chloride is 96 to 106 mEq/L, therefore chloride levels at or above 110 mEq/L usually indicate kidney dysfunction as it is a regulator of chloride concentration. As of now there are no specific symptoms of hyperchloremia; however, it can be influenced by multiple abnormalities that cause a loss of electrolyte-free fluid, loss of hypotonic fluid, or increased administration of sodium chloride. These abnormalities are caused by diarrhea, vomiting, increased sodium chloride intake, renal dysfunction, diuretic use, and diabetes. Hyperchloremia should not be mistaken for hyperchloremic metabolic acidosis as hyperchloremic metabolic acidosis is characterized by two major changes: a decrease in blood pH and bicarbonate levels, as well as an increase in blood chloride levels. Instead those with hyperchloremic metabolic acidosis are usually predisposed to hyperchloremia.

Saline is a mixture of sodium chloride (salt) and water. It has a number of uses in medicine including cleaning wounds, removal and storage of contact lenses, and help with dry eyes. By injection into a vein it is used to treat dehydration such as that from gastroenteritis and diabetic ketoacidosis. Large amounts may result in fluid overload, swelling, acidosis, and high blood sodium. In those with long-standing low blood sodium, excessive use may result in osmotic demyelination syndrome.

Hysteroscopy is the inspection of the uterine cavity by endoscopy with access through the cervix. It allows for the diagnosis of intrauterine pathology and serves as a method for surgical intervention.

Oral rehydration therapy (ORT) is a type of fluid replacement used to prevent and treat dehydration, especially due to diarrhea. It involves drinking water with modest amounts of sugar and salts, specifically sodium and potassium. Oral rehydration therapy can also be given by a nasogastric tube. Therapy should routinely include the use of zinc supplements. Use of oral rehydration therapy has been estimated to decrease the risk of death from diarrhea by up to 93%.

Ringer's lactate solution (RL), also known as sodium lactate solution,Lactated Ringer’s, and Hartmann's solution, is a mixture of sodium chloride, sodium lactate, potassium chloride, and calcium chloride in water. It is used for replacing fluids and electrolytes in those who have low blood volume or low blood pressure. It may also be used to treat metabolic acidosis and to wash the eye following a chemical burn. It is given by intravenous infusion or applied to the affected area.
Fluid balance is an aspect of the homeostasis of organisms in which the amount of water in the organism needs to be controlled, via osmoregulation and behavior, such that the concentrations of electrolytes in the various body fluids are kept within healthy ranges. The core principle of fluid balance is that the amount of water lost from the body must equal the amount of water taken in; for example, in humans, the output must equal the input. Euvolemia is the state of normal body fluid volume, including blood volume, interstitial fluid volume, and intracellular fluid volume; hypovolemia and hypervolemia are imbalances. Water is necessary for all life on Earth. Humans can survive for 4 to 6 weeks without food but only for a few days without water.

Osmotic concentration, formerly known as osmolarity, is the measure of solute concentration, defined as the number of osmoles (Osm) of solute per litre (L) of solution. The osmolarity of a solution is usually expressed as Osm/L, in the same way that the molarity of a solution is expressed as "M". Whereas molarity measures the number of moles of solute per unit volume of solution, osmolarity measures the number of osmoles of solute particles per unit volume of solution. This value allows the measurement of the osmotic pressure of a solution and the determination of how the solvent will diffuse across a semipermeable membrane (osmosis) separating two solutions of different osmotic concentration.
Hyperosmolar syndrome or diabetic hyperosmolar syndrome is a medical emergency caused by a very high blood glucose level.

Insulin aspart, sold under the brand name NovoLog and Fiasp, among others, is a modified type of medical insulin used to treat type 1 and type 2 diabetes. It is generally used by injection under the skin but may also be used by injection into a vein. Maximum effect occurs after about 1–3 hours and lasts for 3–5 hours. Generally a longer-acting insulin like insulin NPH is also needed.
The Ranson criteria form a clinical prediction rule for predicting the prognosis and mortality risk of acute pancreatitis. They were introduced in 1974 by the English-American pancreatic expert and surgeon Dr. John Ranson (1938–1995).
Hemendra Nath Chatterjee was an Indian scientist from West Bengal, who gave a dilute salt and glucose solutions both rectally and orally to a small percentage of pre-selected mildly ill cholera patients. He did not measure intake and output and presented no balance dated confirming absorption. Orally Rehydrated Saline (ORS) for diarrhea management. His paper regarding this finding was published in Lancet of November 1953. In that paper he states that Avomine can stop vomiting during cholera and then oral rehydration is possible. Patients also received a leaf decoction of Coleus aramaticus, a folk anti-diarrheal, along with several other medications. The formulation of the fluid replacement solution was hypotonic sodium chloride, 25 g of glucose and 1000 ml of water. See also:Nalin, DR.https://www.mdpi.com/2414-6366/7/3/50/htm

Salicylate poisoning, also known as aspirin poisoning, is the acute or chronic poisoning with a salicylate such as aspirin. The classic symptoms are ringing in the ears, nausea, abdominal pain, and a fast breathing rate. Early on, these may be subtle, while larger doses may result in fever. Complications can include swelling of the brain or lungs, seizures, low blood sugar, or cardiac arrest.
A balanced salt solution (BSS) is a solution made to a physiological pH and isotonic salt concentration. Solutions most commonly include sodium, potassium, calcium, magnesium, and chloride. Balanced salt solutions are used for washing tissues and cells and are usually combined with other agents to treat the tissues and cells. They provide the cells with water and inorganic ions, while maintaining a physiological pH and osmotic pressure.
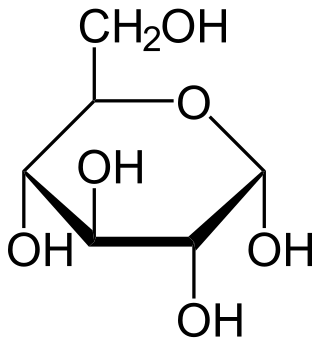
Intravenous sugar solution, also known as dextrose solution, is a mixture of dextrose (glucose) and water. It is used to treat low blood sugar or water loss without electrolyte loss. Water loss without electrolyte loss may occur in fever, hyperthyroidism, high blood calcium, or diabetes insipidus. It is also used in the treatment of high blood potassium, diabetic ketoacidosis, and as part of parenteral nutrition. It is given by injection into a vein.
References
- ↑ Rehydrex from the Swedish official drug catalog Updated 2010-05-04
- ↑ "Rehydrex® med glucos 25 mg/ml - FASS Allmänhet". www.fass.se. Retrieved 2022-05-14.
- ↑ "Rehydrex® med glucos 25 mg/ml - FASS Allmänhet". www.fass.se. Retrieved 2022-05-14.