
Vitamin A is a group of unsaturated nutritional organic compounds that includes retinol, retinal, retinoic acid, and several provitamin A carotenoids. Vitamin A has multiple functions: it is important for growth and development, for the maintenance of the immune system, and for good vision. Vitamin A is needed by the retina of the eye in the form of retinal, which combines with protein opsin to form rhodopsin, the light-absorbing molecule necessary for both low-light and color vision. Vitamin A also functions in a very different role as retinoic acid, which is an important hormone-like growth factor for epithelial and other cells.

Retinol, also known as vitamin A1, is a vitamin found in food and used as a dietary supplement. As a supplement it is used to treat and prevent vitamin A deficiency, especially that which results in xerophthalmia. In regions where deficiency is common, a single large dose is recommended to those at high risk a couple of times a year. It is also used to reduce the risk of complications in those who have measles. It is used by mouth or injection into a muscle.

Rhodopsin is a light-sensitive receptor protein involved in visual phototransduction. It is named after ancient Greek ῥόδον (rhódon) for rose, due to its pinkish color, and ὄψις (ópsis) for sight. Rhodopsin is a biological pigment found in the rods of the retina and is a G-protein-coupled receptor (GPCR). It belongs to opsins. Rhodopsin is extremely sensitive to light, and thus enables vision in low-light conditions. When rhodopsin is exposed to light, it immediately photobleaches. In humans, it is regenerated fully in about 30 minutes, after which rods are more sensitive.

A photoreceptor cell is a specialized type of neuroepithelial cell found in the retina that is capable of visual phototransduction. The great biological importance of photoreceptors is that they convert light into signals that can stimulate biological processes. To be more specific, photoreceptor proteins in the cell absorb photons, triggering a change in the cell's membrane potential.

Rod cells are photoreceptor cells in the retina of the eye that can function in lower light than the other type of visual photoreceptor, cone cells. Rods are usually found concentrated at the outer edges of the retina and are used in peripheral vision. On average, there are approximately 92 million rod cells in the human retina. Rod cells are more sensitive than cone cells and are almost entirely responsible for night vision. However, rods have little role in color vision, which is the main reason why colors are much less apparent in dim light.
In visual physiology, adaptation is the ability of the retina of the eye to adjust to various levels of light. Natural night vision, or scotopic vision, is the ability to see under low-light conditions. In humans, rod cells are exclusively responsible for night vision as cone cells are only able to function at higher illumination levels. Night vision is of lower quality than day vision because it is limited in resolution and colors cannot be discerned; only shades of gray are seen. In order for humans to transition from day to night vision they must undergo a dark adaptation period of up to two hours in which each eye adjusts from a high to a low luminescence "setting", increasing sensitivity hugely, by many orders of magnitude. This adaptation period is different between rod and cone cells and results from the regeneration of photopigments to increase retinal sensitivity. Light adaptation, in contrast, works very quickly, within seconds.

Photopsins are the photoreceptor proteins found in the cone cells of the retina that are the basis of color vision. Iodopsin, the cone pigment system in chicken retina, is a close analog of the visual purple rhodopsin that is used in night vision. Iodopsin consists of the protein component and a bound chromophore, retinal.
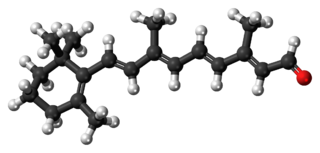
Retinal, also known as retinaldehyde, is a form of vitamin A. It was originally called retinene, and renamed after it was discovered to be vitamin A aldehyde. Retinal is one of the many forms of vitamin A. Retinal is a polyene chromophore, bound to proteins called opsins, and is the chemical basis of animal vision. Retinal allows certain microorganisms to convert light into metabolic energy.
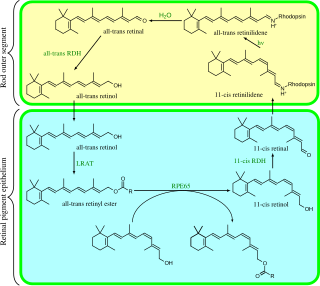
Visual phototransduction is the sensory transduction of the visual system. It is a process by which light is converted into electrical signals in the rod cells, cone cells and photosensitive ganglion cells of the retina of the eye. This cycle was elucidated by George Wald (1906–1997) for which he received the Nobel Prize in 1967. It is so called "Wald's Visual Cycle" after him.

Retinoic acid (used simplified here for all-trans-retinoic acid) is a metabolite of vitamin A1 (all-trans-retinol) that mediates the functions of vitamin A1 required for growth and development. All-trans-retinoic acid is required in chordate animals, which includes all higher animals from fish to humans. During early embryonic development, all-trans-retinoic acid generated in a specific region of the embryo helps determine position along the embryonic anterior/posterior axis by serving as an intercellular signaling molecule that guides development of the posterior portion of the embryo. It acts through Hox genes, which ultimately control anterior/posterior patterning in early developmental stages.

Carotenoid oxygenases are a family of enzymes involved in the cleavage of carotenoids to produce, for example, retinol, commonly known as vitamin A. This family includes an enzyme known as RPE65 which is abundantly expressed in the retinal pigment epithelium where it catalyzed the formation of 11-cis-retinol from all-trans-retinyl esters.

Ruth Hubbard was a professor of biology at Harvard University, where she was the first woman to hold a tenured professorship position in biology.
Retinylidene protein, is a family of proteins that use retinal as a chromophore for light reception. It is the molecular basis for a variety of light-sensing systems from phototaxis in flagellates to eyesight in animals. Retinylidene proteins include all forms of opsin and rhodopsin. While rhodopsin in the narrow sense refers to a dim-light visual pigment found in vertebrates, usually on rod cells, rhodopsin in the broad sense refers any molecule consisting of an opsin and a retinal chromophore in the ground state. When activated by light, the chromophore is isomerized, at which point the molecule as a whole is no longer rhodopsin, but a related molecule such as metarhodopsin. However, it remains a retinylidene protein. The chromophore then separates from the opsin, at which point the bare opsin is a retinylidene protein. Thus, the molecule remains a retinylidene protein throughout the phototransduction cycle.
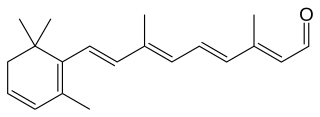
Dehydroretinal, more specifically, 3,4-dehydroretinal, is a derivative metabolite of retinal belonging to the group of vitamin A2 as a retinaldehyde form, besides the endogenously present 3,4-dehydroretinol and 3,4-dehydroretinoic acid.
The bleach and recycle process is used within the retina to ensure that the chromophore 11-cis retinal is present within opsin molecules in sufficient quantities to allow phototransduction to occur. It uses vitamin A (retinol) derivatives.
In enzymology, a retinol dehydrogenase (RDH) (EC 1.1.1.105) is an enzyme that catalyzes the chemical reaction

RPE-retinal G protein-coupled receptor also known as RGR-opsin is a protein that in humans is encoded by the RGR gene.

Retinal pigment epithelium-specific 65 kDa protein, also known as retinoid isomerohydrolase, is an enzyme of the vertebrate visual cycle that is encoded in humans by the RPE65 gene. RPE65 is expressed in the retinal pigment epithelium and is responsible for the conversion of all-trans-retinyl esters to 11-cis-retinol during phototransduction. 11-cis-retinol is then used in visual pigment regeneration in photoreceptor cells. RPE65 belongs to the carotenoid oxygenase family of enzymes.

Retinaldehyde-binding protein 1 (RLBP1) also known as cellular retinaldehyde-binding protein (CRALBP) is a 36-kD water-soluble protein that in humans is encoded by the RLBP1 gene.

Emixustat is a small molecule notable for its establishment of a new class of compounds known as visual cycle modulators (VCMs). Formulated as the hydrochloride salt, emixustat hydrochloride, it is the first synthetic medicinal compound shown to affect retinal disease processes when taken by mouth. Emixustat was invented by the British-American chemist, Ian L. Scott, and is currently in Phase 3 trials for dry, age-related macular degeneration (AMD).
















