
The centromere links a pair of sister chromatids together during cell division. This constricted region of chromosome connects the sister chromatids, creating a short arm (p) and a long arm (q) on the chromatids. During mitosis, spindle fibers attach to the centromere via the kinetochore.
Heterochromatin is a tightly packed form of DNA or condensed DNA, which comes in multiple varieties. These varieties lie on a continuum between the two extremes of constitutive heterochromatin and facultative heterochromatin. Both play a role in the expression of genes. Because it is tightly packed, it was thought to be inaccessible to polymerases and therefore not transcribed; however, according to Volpe et al. (2002), and many other papers since, much of this DNA is in fact transcribed, but it is continuously turned over via RNA-induced transcriptional silencing (RITS). Recent studies with electron microscopy and OsO4 staining reveal that the dense packing is not due to the chromatin.

Schizosaccharomyces pombe, also called "fission yeast", is a species of yeast used in traditional brewing and as a model organism in molecular and cell biology. It is a unicellular eukaryote, whose cells are rod-shaped. Cells typically measure 3 to 4 micrometres in diameter and 7 to 14 micrometres in length. Its genome, which is approximately 14.1 million base pairs, is estimated to contain 4,970 protein-coding genes and at least 450 non-coding RNAs.

A kinetochore is a disc-shaped protein structure associated with duplicated chromatids in eukaryotic cells where the spindle fibers attach during cell division to pull sister chromatids apart. The kinetochore assembles on the centromere and links the chromosome to microtubule polymers from the mitotic spindle during mitosis and meiosis. The term kinetochore was first used in a footnote in a 1934 Cytology book by Lester W. Sharp and commonly accepted in 1936. Sharp's footnote reads: "The convenient term kinetochore has been suggested to the author by J. A. Moore", likely referring to John Alexander Moore who had joined Columbia University as a freshman in 1932.
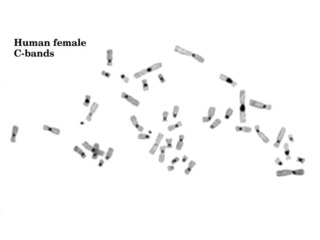
Constitutive heterochromatin domains are regions of DNA found throughout the chromosomes of eukaryotes. The majority of constitutive heterochromatin is found at the pericentromeric regions of chromosomes, but is also found at the telomeres and throughout the chromosomes. In humans there is significantly more constitutive heterochromatin found on chromosomes 1, 9, 16, 19 and Y. Constitutive heterochromatin is composed mainly of high copy number tandem repeats known as satellite repeats, minisatellite and microsatellite repeats, and transposon repeats. In humans these regions account for about 200Mb or 6.5% of the total human genome, but their repeat composition makes them difficult to sequence, so only small regions have been sequenced.
Subtelomeres are segments of DNA between telomeric caps and chromatin.
The Ty5 is a type of retrotransposon native to the Saccharomyces cerevisiae organism.

Schizosaccharomyces is a genus of fission yeasts. The most well-studied species is S. pombe. At present five Schizosaccharomyces species have been described. Like the distantly related Saccharomyces cerevisiae, S. pombe is a significant model organism in the study of eukaryotic cell biology. It is particularly useful in evolutionary studies because it is thought to have diverged from the Saccharomyces cerevisiae lineage between 300 million and 1 billion years ago, and thus provides an evolutionarily distant comparison.
The family of heterochromatin protein 1 (HP1) consists of highly conserved proteins, which have important functions in the cell nucleus. These functions include gene repression by heterochromatin formation, transcriptional activation, regulation of binding of cohesion complexes to centromeres, sequestration of genes to the nuclear periphery, transcriptional arrest, maintenance of heterochromatin integrity, gene repression at the single nucleosome level, gene repression by heterochromatization of euchromatin, and DNA repair. HP1 proteins are fundamental units of heterochromatin packaging that are enriched at the centromeres and telomeres of nearly all eukaryotic chromosomes with the notable exception of budding yeast, in which a yeast-specific silencing complex of SIR proteins serve a similar function. Members of the HP1 family are characterized by an N-terminal chromodomain and a C-terminal chromoshadow domain, separated by a hinge region. HP1 is also found at some euchromatic sites, where its binding can correlate with either gene repression or gene activation. HP1 was originally discovered by Tharappel C James and Sarah Elgin in 1986 as a factor in the phenomenon known as position effect variegation in Drosophila melanogaster.
RNA-induced transcriptional silencing (RITS) is a form of RNA interference by which short RNA molecules – such as small interfering RNA (siRNA) – trigger the downregulation of transcription of a particular gene or genomic region. This is usually accomplished by posttranslational modification of histone tails which target the genomic region for heterochromatin formation. The protein complex that binds to siRNAs and interacts with the methylated lysine 9 residue of histones H3 (H3K9me2) is the RITS complex.

Aurora kinase B is a protein that functions in the attachment of the mitotic spindle to the centromere and in cytokinesis.

Centromere protein B also known as major centromere autoantigen B is an autoantigen protein of the cell nucleus. In humans, centromere protein B is encoded by the CENPB gene.

Centromere protein A, also known as CENPA, is a protein which in humans is encoded by the CENPA gene. CENPA is a histone H3 variant which is the critical factor determining the kinetochore position(s) on each chromosome in most eukaryotes including humans.

Centromere protein H is a protein that in humans is encoded by the CENPH gene. It is involved in the assembly of kinetochore proteins, mitotic progression and chromosome segregation.

Telomeric repeat–containing RNA (TERRA) is a long non-coding RNA transcribed from telomeres - repetitive nucleotide regions found on the ends of chromosomes that function to protect DNA from deterioration or fusion with neighboring chromosomes. TERRA has been shown to be ubiquitously expressed in almost all cell types containing linear chromosomes - including humans, mice, and yeasts. While the exact function of TERRA is still an active area of research, it is generally believed to play a role in regulating telomerase activity as well as maintaining the heterochromatic state at the ends of chromosomes. TERRA interaction with other associated telomeric proteins has also been shown to help regulate telomere integrity in a length-dependent manner.
SilentInformationRegulator (SIR) proteins are involved in regulating gene expression. SIR proteins organize heterochromatin near telomeres, ribosomal DNA (rDNA), and at silent loci including hidden mating type loci in yeast. The SIR family of genes encodes catalytic and non-catalytic proteins that are involved in de-acetylation of histone tails and the subsequent condensation of chromatin around a SIR protein scaffold. Some SIR family members are conserved from yeast to humans.

Neocentromeres are new centromeres that form at a place on the chromosome that is usually not centromeric. They typically arise due to disruption of the normal centromere. These neocentromeres should not be confused with “knobs”, which were also described as “neocentromeres” in maize in the 1950s. Unlike most normal centromeres, neocentromeres do not contain satellite sequences that are highly repetitive but instead consist of unique sequences. Despite this, most neocentromeres are still able to carry out the functions of normal centromeres in regulating chromosome segregation and inheritance. This raises many questions on what is necessary versus what is sufficient for constituting a centromere.

Thomas Jenuwein is a German scientist working in the fields of epigenetics, chromatin biology, gene regulation and genome function.

Kaustuv Sanyal is an Indian molecular biologist, mycologist and Director of Bose Institute in Kolkata. He is a professor at the Molecular Biology and Genetics Unit of the Jawaharlal Nehru Centre for Advanced Scientific Research (JNCASR). He is known for his molecular and genetic studies of pathogenic yeasts such as Candida and Cryptococcus). An alumnus of Bidhan Chandra Krishi Viswavidyalaya and Madurai Kamaraj University from where he earned a BSc in agriculture and MSc in biotechnology respectively, Sanyal did his doctoral studies at Bose Institute to secure a PhD in Yeast genetics. He moved to the University of California, Santa Barbara, USA to work in the laboratory of John Carbon on the discovery of centromeres in Candida albicans. He joined JNCASR in 2005. He is a member of the Faculty of 1000 in the disciplines of Microbial Evolution and Genomics and has delivered invited speeches which include the Gordon Research Conference, EMBO conferences on comparative genomics and kinetochores. The Department of Biotechnology of the Government of India awarded him the National Bioscience Award for Career Development, one of the highest Indian science awards, for his contributions to biosciences, in 2012. He has also been awarded with the prestigious Tata Innovation Fellowship in 2017. The National Academy of Sciences, India elected him as a fellow in 2014. He is also an elected fellow of Indian Academy of Sciences (2017), and the Indian National Science Academy (2018). In 2019, he has been elected to Fellowship in the American Academy of Microbiology (AAM), the honorific leadership group within the American Society for Microbiology. He was awarded the J.C. Bose National Fellowship in 2020.
Holocentric chromosomes are chromosomes that possess multiple kinetochores along their length rather than the single centromere typical of other chromosomes. They were first described in cytogenetic experiments in 1935. Since this first observation, the term holocentric chromosome has referred to chromosomes that: i) lack the primary constriction corresponding to the centromere observed in monocentric chromosomes; and ii) possess multiple kinetochores dispersed along the entire chromosomal axis, such that microtubules bind to the chromosome along its entire length and move broadside to the pole from the metaphase plate. Holocentric chromosomes are also termed holokinetic, because, during cell division, the sister chromatids move apart in parallel and do not form the classical V-shaped figures typical of monocentric chromosomes.












