Related Research Articles

Phosphatidylinositol consists of a family of lipids made of a phosphate group, two fatty acid chains, and one inositol molecule. They represent a class of the phosphatidylglycerides. Typically phosphatidylinositols form a minor component on the cytosolic side of eukaryotic cell membranes. The phosphate group gives the molecules a negative charge at physiological pH.

Phosphoinositide phospholipase C is a family of eukaryotic intracellular enzymes that play an important role in signal transduction processes. These enzymes belong to a larger superfamily of Phospholipase C. Other families of phospholipase C enzymes have been identified in bacteria and trypanosomes. Phospholipases C are phosphodiesterases.
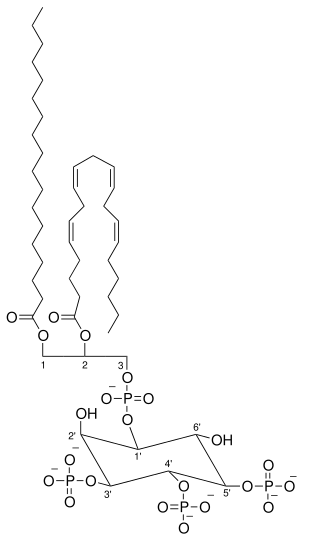
Phosphatidylinositol (3,4,5)-trisphosphate (PtdIns(3,4,5)P3), abbreviated PIP3, is the product of the class I phosphoinositide 3-kinases' (PI 3-kinases) phosphorylation of phosphatidylinositol (4,5)-bisphosphate (PIP2). It is a phospholipid that resides on the plasma membrane.

Phosphoinositide 3-kinases (PI3Ks), also called phosphatidylinositol 3-kinases, are a family of enzymes involved in cellular functions such as cell growth, proliferation, differentiation, motility, survival and intracellular trafficking, which in turn are involved in cancer.
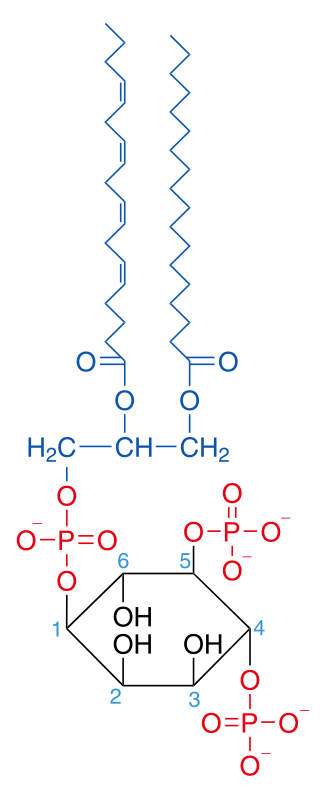
Phosphatidylinositol 4,5-bisphosphate or PtdIns(4,5)P2, also known simply as PIP2 or PI(4,5)P2, is a minor phospholipid component of cell membranes. PtdIns(4,5)P2 is enriched at the plasma membrane where it is a substrate for a number of important signaling proteins. PIP2 also forms lipid clusters that sort proteins.

Phosphatidylinositol (3,4)-bisphosphate is a minor phospholipid component of cell membranes, yet an important second messenger. The generation of PtdIns(3,4)P2 at the plasma membrane activates a number of important cell signaling pathways.

Phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit delta isoform also known as phosphoinositide 3-kinase (PI3K) delta isoform or p110δ is an enzyme that in humans is encoded by the PIK3CD gene.

Phosphatidylinositol 3,5-bisphosphate is one of the seven phosphoinositides found in eukaryotic cell membranes. In quiescent cells, the PtdIns(3,5)P2 levels, typically quantified by HPLC, are the lowest amongst the constitutively present phosphoinositides. They are approximately 3 to 5-fold lower as compared to PtdIns3P and PtdIns5P levels, and more than 100-fold lower than the abundant PtdIns4P and PtdIns(4,5)P2. PtdIns(3,5)P2 was first reported to occur in mouse fibroblasts and budding yeast S. cerevisiae in 1997. In S. cerevisiae PtdIns(3,5)P2 levels increase dramatically during hyperosmotic shock. The response to hyperosmotic challenge is not conserved in most tested mammalian cells except for differentiated 3T3L1 adipocytes.
Phosphatidylinositol phosphate kinases (PIPK) are kinases that phosphorylate the phosphoinositides PtdInsP and PtdInsP2 that are derivatives of phosphatidylinositol (PtdIns). It has been found that PtdIns is only phosphorylated on three (3,4,5) of its five hydroxyl groups, possibly because D-2 and D-6 hydroxyl groups cannot be phosphorylated because of steric hindrance. All 7 combinations of phosphorylated PtdIns have been found in animals, all except PtdIns(3,4,5)P3 have been found in plants.
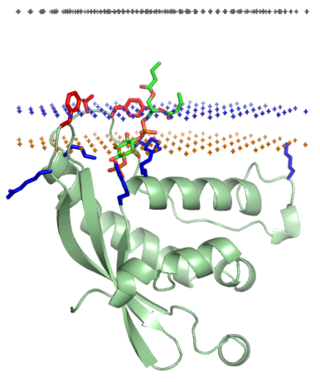
The PX domain is a phosphoinositide-binding structural domain involved in targeting of proteins to cell membranes.
In enzymology, a phosphatidylinositol-4,5-bisphosphate 3-kinase is an enzyme that catalyzes the chemical reaction:
Arf-GAP with dual PH domain-containing protein 1 is a protein that in humans is encoded by the ADAP1 gene.

Phosphatidylinositol 3,4,5-trisphosphate-dependent Rac exchanger 1 protein is a protein that in humans is encoded by the PREX1 gene.
The Akt signaling pathway or PI3K-Akt signaling pathway is a signal transduction pathway that promotes survival and growth in response to extracellular signals. Key proteins involved are PI3K and Akt.

In the field of biochemistry, PDPK1 refers to the protein 3-phosphoinositide-dependent protein kinase-1, an enzyme which is encoded by the PDPK1 gene in humans. It is implicated in the development and progression of melanomas.
Phosphatidylinositol 5-phosphate (PtdIns5P) is a phosphoinositide, one of the phosphorylated derivatives of phosphatidylinositol (PtdIns), that are well-established membrane-anchored regulatory molecules. Phosphoinositides participate in signaling events that control cytoskeletal dynamics, intracellular membrane trafficking, cell proliferation and many other cellular functions. Generally, phosphoinositides transduce signals by recruiting specific phosphoinositide-binding proteins to intracellular membranes.
Lewis C. Cantley is an American cell biologist and biochemist who has made significant advances to the understanding of cancer metabolism. Among his most notable contributions are the discovery and study of the enzyme PI-3-kinase, now known to be important to understanding cancer and diabetes mellitus. He is currently Meyer Director and Professor of Cancer Biology at the Sandra and Edward Meyer Cancer Center at Weill Cornell Medicine in New York City. He was formerly a professor in the Departments of Systems Biology and Medicine at Harvard Medical School, and the Director of Cancer Research at the Beth Israel Deaconess Medical Center, in Boston, Massachusetts. In 2016, he was elected Chairman of the Board for the Hope Funds for Cancer Research.
72 kDa inositol polyphosphate 5-phosphatase, also known as phosphatidylinositol-4,5-bisphosphate 5-phosphatase or Pharbin, is an enzyme that in humans is encoded by the INPP5E gene.
Phosphatidylinositol-3,4,5-trisphosphate 5-phosphatase is an enzyme with systematic name 1-phosphatidyl-1D-myo-inositol-3,4,5-trisphosphate 5-phosphohydrolase, that has two isoforms: SHIP1 and SHIP2 (INPPL1).
Phillip (Phill) Thomas Hawkins FRS is a molecular biologist, senior group leader at the Babraham Institute.
References
- ↑ royalsociety.org http://royalsociety.org/people/leonard-stephens/ . Retrieved 12 January 2014.
{{cite web}}: Missing or empty|title=(help)[ title missing ] - ↑ Stephens, L.R.; Hughes, K.T.; Irvine, R.F. (1991). "Pathway of phosphatidylinositol(3,4,5)-trisphosphate synthesis in activated neutrophils". Nature. 351 (6321): 33–39. Bibcode:1991Natur.351...33S. doi:10.1038/351033a0. PMID 1851250. S2CID 12322154.
- ↑ Hawkins, P.T.; Jackson, T.R.; Stephens, L.R. (1992). "Platelet-derived growth factor stimulates synthesis of Ptdlns(3,4,5)P3 by activating a Ptdlns(4,5)P2 3-OH kinase". Nature. 358 (6382): 157–159. Bibcode:1992Natur.358..157H. doi:10.1038/358157a0. PMID 1319558. S2CID 4310878.
- ↑ Stephens, L.; Smrcka, A.; Cooke, F.T.; Jackson, T.R.; Sternweis, P.C.; Hawkins, P.T. (1994). "A novel phosphoinositide 3 kinase activity in myeloid-derived cells is activated by G protein βγ subunits". Cell. 77 (1): 83–93. doi:10.1016/0092-8674(94)90237-2. PMID 8156600. S2CID 53255676.
- ↑ Stephens, L.R.; Anderson, K.; Stokoe, D.; Erdjument-Bromage, H.; Painter, G.F.; Holmes, A.B.; Gaffney, P.R.J.; Reese, C.B.; McCormick, F.; Tempst, P.; Coadwell, J.; Hawkins, P.T. (1998). "Protein Kinase B Kinases That Mediate Phosphatidylinositol 3,4,5-Trisphosphate-Dependent Activation of Protein Kinase B.". Science. 279 (5351): 710–714. Bibcode:1998Sci...279..710S. doi:10.1126/science.279.5351.710. PMID 9445477.
- ↑ Stokoe, D; Stephens, L. R.; Copeland, T; Gaffney, P. R.; Reese, C. B.; Painter, G. F.; Holmes, A. B.; McCormick, F; Hawkins, P. T. (1997). "Dual role of phosphatidylinositol-3,4,5-trisphosphate in the activation of protein kinase B". Science. 277 (5325): 567–70. doi:10.1126/science.277.5325.567. PMID 9228007.