Related Research Articles
Inositol trisphosphate or inositol 1,4,5-trisphosphate abbreviated InsP3 or Ins3P or IP3 is an inositol phosphate signaling molecule. It is made by hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2), a phospholipid that is located in the plasma membrane, by phospholipase C (PLC). Together with diacylglycerol (DAG), IP3 is a second messenger molecule used in signal transduction in biological cells. While DAG stays inside the membrane, IP3 is soluble and diffuses through the cell, where it binds to its receptor, which is a calcium channel located in the endoplasmic reticulum. When IP3 binds its receptor, calcium is released into the cytosol, thereby activating various calcium regulated intracellular signals.
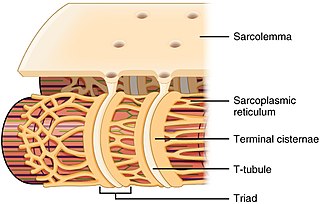
The sarcoplasmic reticulum (SR) is a membrane-bound structure found within muscle cells that is similar to the smooth endoplasmic reticulum in other cells. The main function of the SR is to store calcium ions (Ca2+). Calcium ion levels are kept relatively constant, with the concentration of calcium ions within a cell being 10,000 times smaller than the concentration of calcium ions outside the cell. This means that small increases in calcium ions within the cell are easily detected and can bring about important cellular changes (the calcium is said to be a second messenger). Calcium is used to make calcium carbonate (found in chalk) and calcium phosphate, two compounds that the body uses to make teeth and bones. This means that too much calcium within the cells can lead to hardening (calcification) of certain intracellular structures, including the mitochondria, leading to cell death. Therefore, it is vital that calcium ion levels are controlled tightly, and can be released into the cell when necessary and then removed from the cell.

Inositol trisphosphate receptor (InsP3R) is a membrane glycoprotein complex acting as a Ca2+ channel activated by inositol trisphosphate (InsP3). InsP3R is very diverse among organisms, and is necessary for the control of cellular and physiological processes including cell division, cell proliferation, apoptosis, fertilization, development, behavior, learning and memory. Inositol triphosphate receptor represents a dominant second messenger leading to the release of Ca2+ from intracellular store sites. There is strong evidence suggesting that the InsP3R plays an important role in the conversion of external stimuli to intracellular Ca2+ signals characterized by complex patterns relative to both space and time, such as Ca2+ waves and oscillations.

Ligand-gated ion channels (LICs, LGIC), also commonly referred to as ionotropic receptors, are a group of transmembrane ion-channel proteins which open to allow ions such as Na+, K+, Ca2+, and/or Cl− to pass through the membrane in response to the binding of a chemical messenger (i.e. a ligand), such as a neurotransmitter.
Voltage-gated calcium channels (VGCCs), also known as voltage-dependent calcium channels (VDCCs), are a group of voltage-gated ion channels found in the membrane of excitable cells (e.g., muscle, glial cells, neurons, etc.) with a permeability to the calcium ion Ca2+. These channels are slightly permeable to sodium ions, so they are also called Ca2+-Na+ channels, but their permeability to calcium is about 1000-fold greater than to sodium under normal physiological conditions.
Ryanodine receptors form a class of intracellular calcium channels in various forms of excitable animal tissue like muscles and neurons. There are three major isoforms of the ryanodine receptor, which are found in different tissues and participate in different signaling pathways involving calcium release from intracellular organelles. The RYR2 ryanodine receptor isoform is the major cellular mediator of calcium-induced calcium release (CICR) in animal cells.
The formyl peptide receptors (FPR) belong to a class of G protein-coupled receptors involved in chemotaxis. In humans, there are three formyl peptide receptor isoforms, each encoded by a separate gene that are named FPR1, FPR2, and FPR3. These receptors were originally identified by their ability to bind N-formyl peptides such as N-formylmethionine produced by the degradation of either bacterial or host cells. Hence formyl peptide receptors are involved in mediating immune cell response to infection. These receptors may also act to suppress the immune system under certain conditions. The close phylogenetic relation of signaling in chemotaxis and olfaction was recently proved by detection formyl peptide receptor like proteins as a distinct family of vomeronasal organ chemosensors in mice.

Peptidyl-prolyl cis-trans isomerase FKBP1A is an enzyme that in humans is encoded by the FKBP1A gene. It is also commonly referred to as FKBP-12 or FKBP12 and is a member of a family of FK506-binding proteins (FKBPs).

Inositol 1,4,5-trisphosphate receptor type 1 is a protein that in humans is encoded by the ITPR1 gene.

Ryanodine receptor 2 (RYR2) is one of a class of ryanodine receptors and a protein found primarily in cardiac muscle. In humans, it is encoded by the RYR2 gene. In the process of cardiac calcium-induced calcium release, RYR2 is the major mediator for sarcoplasmic release of stored calcium ions.

Cav1.1 also known as the calcium channel, voltage-dependent, L type, alpha 1S subunit, (CACNA1S), is a protein which in humans is encoded by the CACNA1S gene. It is also known as CACNL1A3 and the dihydropyridine receptor.
Peptidyl-prolyl cis-trans isomerase FKBP1B is an enzyme that in humans is encoded by the FKBP1B gene.

Ryanodine receptor 1 (RYR-1) also known as skeletal muscle calcium release channel or skeletal muscle-type ryanodine receptor is one of a class of ryanodine receptors and a protein found primarily in skeletal muscle. In humans, it is encoded by the RYR1 gene.
Ankyrin-2, also known as Ankyrin-B, and Brain ankyrin, is a protein which in humans is encoded by the ANK2 gene. Ankyrin-2 is ubiquitously expressed, but shows high expression in cardiac muscle. Ankyrin-2 plays an essential role in the localization and membrane stabilization of ion transporters and ion channels in cardiomyocytes, as well as in costamere structures. Mutations in ANK2 cause a dominantly-inherited, cardiac arrhythmia syndrome known as long QT syndrome 4 as well as sick sinus syndrome; mutations have also been associated to a lesser degree with hypertrophic cardiomyopathy. Alterations in ankyrin-2 expression levels are observed in human heart failure.

Inositol 1,4,5-trisphosphate receptor, type 3, also known as ITPR3, is a protein which in humans is encoded by the ITPR3 gene. The protein encoded by this gene is both a receptor for inositol triphosphate and a calcium channel.

Ryanodine receptor 3 is one of a class of ryanodine receptors and a protein that in humans is encoded by the RYR3 gene. The protein encoded by this gene is both a calcium channel and a receptor for the plant alkaloid ryanodine. RYR3 and RYR1 control the resting calcium ion concentration in skeletal muscle.
Imperatoxin I (IpTx) is a peptide toxin derived from the venom of the African scorpion Pandinus imperator.
JTV-519 (K201) is a 1,4-benzothiazepine derivative that interacts with many cellular targets. It has many structural similarities to diltiazem, a Ca2+ channel blocker used for treatment of hypertension, angina pectoris and some types of arrhythmias. JTV-519 acts in the sarcoplasmic reticulum (SR) of cardiac myocytes by binding to and stabilizing the ryanodine receptor (RyR2) in its closed state. It can be used in the treatment of cardiac arrhythmias, heart failure, catecholaminergic polymorphic ventricular tachycardia (CPVT) and store overload-induced Ca2+ release (SOICR). Currently, this drug has only been tested on animals and its side effects are still unknown. As research continues, some studies have also found a dose-dependent response; where there is no improvement seen in failing hearts at 0.3 μM and a decline in response at 1 μM.
The transient receptor potential Ca2+ channel (TRP-CC) family (TC# 1.A.4) is a member of the voltage-gated ion channel (VIC) superfamily and consists of cation channels conserved from worms to humans. The TRP-CC family also consists of seven subfamilies (TRPC, TRPV, TRPM, TRPN, TRPA, TRPP, and TRPML) based on their amino acid sequence homology:
- the canonical or classic TRPs,
- the vanilloid receptor TRPs,
- the melastatin or long TRPs,
- ankyrin (whose only member is the transmembrane protein 1 [TRPA1])
- TRPN after the nonmechanoreceptor potential C (nonpC), and the more distant cousins,
- the polycystins
- and mucolipins.
The testis-enhanced gene transcript (TEGT) family includes the testis-enhanced gene transcript proteins of mammals, which are expressed at high levels in the testis, the putative glutamate/aspartate binding proteins of plants and animals, the YccA protein of Escherichia coli and the YetJ protein of Bacillus subtilis. These proteins are about 200-250 residues in length and exhibit 7 TMSs.
References
- ↑ Beutner G, Sharma VK, Giovannucci DR, Yule DI, Sheu SS (June 2001). "Identification of a ryanodine receptor in rat heart mitochondria". The Journal of Biological Chemistry. 276 (24): 21482–8. doi:10.1074/jbc.M101486200. PMID 11297554.
- ↑ Sun QA, Hess DT, Nogueira L, Yong S, Bowles DE, Eu J, Laurita KR, Meissner G, Stamler JS (September 2011). "Oxygen-coupled redox regulation of the skeletal muscle ryanodine receptor-Ca2+ release channel by NADPH oxidase 4". Proceedings of the National Academy of Sciences of the United States of America. 108 (38): 16098–103. Bibcode:2011PNAS..10816098S. doi: 10.1073/pnas.1109546108 . PMC 3179127 . PMID 21896730.
- ↑ Santulli, Gaetano; Marks, Andrew (2015). "Essential Roles of Intracellular Calcium Release Channels in Muscle, Brain, Metabolism, and Aging". Current Molecular Pharmacology. 8 (2): 206–222. doi:10.2174/1874467208666150507105105. ISSN 1874-4672. PMID 25966694.
- ↑ Thomas NL, George CH, Williams AJ, Lai FA (November 2007). "Ryanodine receptor mutations in arrhythmias: advances in understanding the mechanisms of channel dysfunction". Biochemical Society Transactions. 35 (Pt 5): 946–51. doi:10.1042/BST0350946. PMID 17956252.
- ↑ Laver DR, Hamada T, Fessenden JD, Ikemoto N (December 2007). "The ryanodine receptor pore blocker neomycin also inhibits channel activity via a previously undescribed high-affinity Ca(2+) binding site". The Journal of Membrane Biology. 220 (1–3): 11–20. doi:10.1007/s00232-007-9067-3. PMID 17879109. S2CID 38255566.
- ↑ "1.A.3 The Ryanodine-Inositol 1,4,5-triphosphate Receptor Ca2+ Channel (RIR-CaC) Family". TCDB. Retrieved 2016-04-10.
- ↑ Bosanac I, Alattia JR, Mal TK, Chan J, Talarico S, Tong FK, Tong KI, Yoshikawa F, Furuichi T, Iwai M, Michikawa T, Mikoshiba K, Ikura M (December 2002). "Structure of the inositol 1,4,5-trisphosphate receptor binding core in complex with its ligand". Nature. 420 (6916): 696–700. Bibcode:2002Natur.420..696B. doi:10.1038/nature01268. PMID 12442173. S2CID 4422308.
- ↑ Seo MD, Velamakanni S, Ishiyama N, Stathopulos PB, Rossi AM, Khan SA, Dale P, Li C, Ames JB, Ikura M, Taylor CW (January 2012). "Structural and functional conservation of key domains in InsP3 and ryanodine receptors". Nature. 483 (7387): 108–12. Bibcode:2012Natur.483..108S. doi:10.1038/nature10751. PMC 3378505 . PMID 22286060.
- ↑ Mio K, Ogura T, Sato C (May 2008). "Structure of six-transmembrane cation channels revealed by single-particle analysis from electron microscopic images". Journal of Synchrotron Radiation. 15 (Pt 3): 211–4. doi:10.1107/S0909049508004640. PMC 2394823 . PMID 18421141.
- ↑ Du GG, Sandhu B, Khanna VK, Guo XH, MacLennan DH (December 2002). "Topology of the Ca2+ release channel of skeletal muscle sarcoplasmic reticulum (RyR1)". Proceedings of the National Academy of Sciences of the United States of America. 99 (26): 16725–30. Bibcode:2002PNAS...9916725D. doi: 10.1073/pnas.012688999 . PMC 139211 . PMID 12486242.
- ↑ Gaburjakova M, Gaburjakova J, Reiken S, Huang F, Marx SO, Rosemblit N, Marks AR (May 2001). "FKBP12 binding modulates ryanodine receptor channel gating". The Journal of Biological Chemistry. 276 (20): 16931–5. doi: 10.1074/jbc.M100856200 . PMID 11279144.
- ↑ Mikoshiba, Katsuhiko; Furuichi, Teiichi; Miyawaki, Atsushi (1997-01-01). "IP3-sensitive calcium channel". In Lee, A. G. (ed.). Transmembrane Receptors and Channels. Transmembrane Receptors and Channels. Vol. 6. JAI. pp. 273–289. doi:10.1016/s1874-5342(96)80040-7. ISBN 9781559386630.
- ↑ Lur G, Sherwood MW, Ebisui E, Haynes L, Feske S, Sutton R, Burgoyne RD, Mikoshiba K, Petersen OH, Tepikin AV (June 2011). "InsP₃receptors and Orai channels in pancreatic acinar cells: co-localization and its consequences". The Biochemical Journal. 436 (2): 231–9. doi:10.1042/BJ20110083. PMC 3262233 . PMID 21568942.
- 1 2 Schug ZT, da Fonseca PC, Bhanumathy CD, Wagner L, Zhang X, Bailey B, Morris EP, Yule DI, Joseph SK (February 2008). "Molecular characterization of the inositol 1,4,5-trisphosphate receptor pore-forming segment". The Journal of Biological Chemistry. 283 (5): 2939–48. doi: 10.1074/jbc.M706645200 . PMID 18025085.
As of this edit, this article uses content from "1.A.3 The Ryanodine-Inositol 1,4,5-triphosphate Receptor Ca2+ Channel (RIR-CaC) Family" , which is licensed in a way that permits reuse under the Creative Commons Attribution-ShareAlike 3.0 Unported License, but not under the GFDL. All relevant terms must be followed.