Selenoprotein N is a protein that in humans is encoded by the SEPN1 gene. [5] [6]
Selenoprotein N is a protein that in humans is encoded by the SEPN1 gene. [5] [6]
This gene encodes a selenoprotein, which contains a selenocysteine (Sec) residue at its active site. The selenocysteine is encoded by the UGA codon that normally signals translation termination. The 3' UTR of selenoprotein genes have a common stem-loop structure, the sec insertion sequence (SECIS), that is necessary for the recognition of UGA as a Sec codon rather than as a stop signal. Pathogenic Mutations in SEPN1 gene (SELENON) can cause the classical phenotype of multiminicore disease and congenital muscular dystrophy with spinal rigidity and restrictive respiratory syndrome known as SEPN1-related congenital muscular dystrophy or rigid spine syndrome. Two alternatively spliced transcript variants encoding distinct isoforms have been found for this gene. [6]
In molecular biology a selenoprotein is any protein that includes a selenocysteine amino acid residue. Among functionally characterized selenoproteins are five glutathione peroxidases (GPX) and three thioredoxin reductases, (TrxR/TXNRD) which both contain only one Sec. Selenoprotein P is the most common selenoprotein found in the plasma. It is unusual because in humans it contains 10 Sec residues, which are split into two domains, a longer N-terminal domain that contains 1 Sec, and a shorter C-terminal domain that contains 9 Sec. The longer N-terminal domain is likely an enzymatic domain, and the shorter C-terminal domain is likely a means of safely transporting the very reactive selenium atom throughout the body.
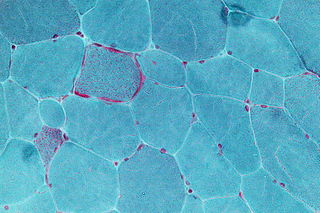
In medicine, a muscle biopsy is a procedure in which a piece of muscle tissue is removed from an organism and examined microscopically. A muscle biopsy can lead to the discovery of problems with the nervous system, connective tissue, vascular system, or musculoskeletal system.
Derek Blake was, until 2007, the Isobel Laing Post-Doctoral Fellow in Biomedical Sciences, and the Wellcome Trust Senior Fellow in Basic Biomedical Science, Oriel College, Oxford.

Centronuclear myopathies (CNM) are a group of congenital myopathies where cell nuclei are abnormally located in the center of muscle cells instead of their normal location at the periphery.

Congenital muscular dystrophies are autosomal recessively-inherited muscle diseases. They are a group of heterogeneous disorders characterized by muscle weakness which is present at birth and the different changes on muscle biopsy that ranges from myopathic to overtly dystrophic due to the age at which the biopsy takes place.

Emery–Dreifuss muscular dystrophy (EDMD) is a type of muscular dystrophy, a group of heritable diseases that cause progressive impairment of muscles. EDMD affects muscles used for movement, causing atrophy, weakness and contractures. It almost always affects the heart, causing abnormal rhythms, heart failure, or sudden cardiac death. It is rare, affecting 0.39 per 100,000 people. It is named after Alan Eglin H. Emery and Fritz E. Dreifuss.

Distal myopathy is a group of rare genetic disorders that cause muscle damage and weakness, predominantly in the hands and/or feet. Mutation of many different genes can be causative. Many types involve dysferlin.

Bethlem myopathy is predominantly an autosomal dominant myopathy, classified as a congenital form of limb-girdle muscular dystrophy. There are two types of Bethlem myopathy, based on which type of collagen is affected.

Fukutin is a eukaryotic protein necessary for the maintenance of muscle integrity, cortical histogenesis, and normal ocular development. Mutations in the fukutin gene have been shown to result in Fukuyama congenital muscular dystrophy (FCMD) characterised by brain malformation - one of the most common autosomal-recessive disorders in Japan. In humans this protein is encoded by the FCMD gene, located on chromosome 9q31. Human fukutin exhibits a length of 461 amino acids and a predicted molecular mass of 53.7 kDa.
Calpain-3 is a protein that in humans is encoded by the CAPN3 gene.

Laminin subunit alpha-2 is a protein that in humans is encoded by the LAMA2 gene.

Alpha-7 integrin is a protein that in humans is encoded by the ITGA7 gene. Alpha-7 integrin is critical for modulating cell-matrix interactions. Alpha-7 integrin is highly expressed in cardiac muscle, skeletal muscle and smooth muscle cells, and localizes to Z-disc and costamere structures. Mutations in ITGA7 have been associated with congenital myopathies and noncompaction cardiomyopathy, and altered expression levels of alpha-7 integrin have been identified in various forms of muscular dystrophy.

Polyadenylate-binding protein 2 (PABP-2) also known as polyadenylate-binding nuclear protein 1 (PABPN1) is a protein that in humans is encoded by the PABPN1 gene. PABN1 is a member of a larger family of poly(A)-binding proteins in the human genome.

Collagen alpha-3(VI) chain is a protein that in humans is encoded by the COL6A3 gene. This protein is an alpha chain of type VI collagen that aids in microfibril formation. As part of type VI collagen, this protein has been implicated in Bethlem myopathy, Ullrich congenital muscular dystrophy (UCMD), and other diseases related to muscle and connective tissue.

Alpha-sarcoglycan is a protein that in humans is encoded by the SGCA gene.

Myotilin is a protein that in humans is encoded by the MYOT gene. Myotilin also known as TTID is a muscle protein that is found within the Z-disc of sarcomeres.

Ullrich congenital muscular dystrophy (UCMD) is a form of congenital muscular dystrophy. There are two forms: UCMD1 and UCMD2.
Collagen VI (ColVI) is a type of collagen primarily associated with the extracellular matrix of skeletal muscle. ColVI maintains regularity in muscle function and stabilizes the cell membrane. It is synthesized by a complex, multistep pathway that leads to the formation of a unique network of linked microfilaments located in the extracellular matrix (ECM). ColVI plays a vital role in numerous cell types, including chondrocytes, neurons, myocytes, fibroblasts, and cardiomyocytes. ColVI molecules are made up of three alpha chains: α1(VI), α2(VI), and α3(VI). It is encoded by 6 genes: COL6A1, COL6A2, COL6A3, COL6A4, COL6A5, and COL6A6. The chain lengths of α1(VI) and α2(VI) are about 1,000 amino acids. The chain length of α3(VI) is roughly a third larger than those of α1(VI) and α2(VI), and it consists of several spliced variants within the range of 2,500 to 3,100 amino acids.
Michel Fardeau, is a medical researcher in medical pathology, pioneering founder in France of myology, a medical discipline treating diseases of the neuromuscular system. He was also a full professor at the Conservatoire National des Arts et Métiers in a chair dedicated to the social integration of disabled people.
Rigid spine syndrome, also known as congenital muscular dystrophy with rigidity of the spine (CMARS), is a rare and often debilitating neuromuscular disorder. It is characterized by progressive muscle stiffness and rigidity, particularly in the spine, which can severely limit mobility and impact quality of life. This condition is typically present from birth or early childhood and tends to worsen over time.