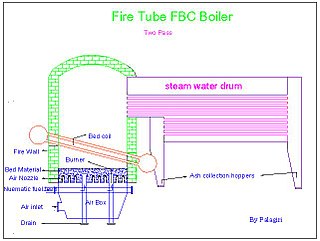
Fluidized bed combustion (FBC) is a combustion technology used to burn solid fuels.

Exhaust gas or flue gas is emitted as a result of the combustion of fuels such as natural gas, gasoline, petrol, biodiesel blends, diesel fuel, fuel oil, or coal. According to the type of engine, it is discharged into the atmosphere through an exhaust pipe, flue gas stack, or propelling nozzle. It often disperses downwind in a pattern called an exhaust plume.
Steam reforming or steam methane reforming is a chemical synthesis for producing syngas from hydrocarbons such as natural gas. This is achieved in a reformer which reacts steam at high temperature and pressure with methane in the presence of a nickel catalyst. The steam methane reformer is widely used in industry to make hydrogen.
Coal gasification is the process of producing syngas–a mixture consisting primarily of carbon monoxide (CO), hydrogen (H2), carbon dioxide (CO2), natural gas (CH4), and water vapour (H2O)–from coal and water, air and/or oxygen.

Flue-gas desulfurization (FGD) is a set of technologies used to remove sulfur dioxide from exhaust flue gases of fossil-fuel power plants, and from the emissions of other sulfur oxide emitting processes such as waste incineration.

A fossil fuel power station is a thermal power station which burns a fossil fuel, such as coal or natural gas, to produce electricity. Fossil fuel power stations have machinery to convert the heat energy of combustion into mechanical energy, which then operates an electrical generator. The prime mover may be a steam turbine, a gas turbine or, in small plants, a reciprocating gas engine. All plants use the energy extracted from expanding gas, either steam or combustion gases. Although different energy conversion methods exist, all thermal power station conversion methods have efficiency limited by the Carnot efficiency and therefore produce waste heat.

The Claus process is the most significant gas desulfurizing process, recovering elemental sulfur from gaseous hydrogen sulfide. First patented in 1883 by the chemist Carl Friedrich Claus, the Claus process has become the industry standard. C. F. Claus was born in Kassel in the German State of Hessen in 1827, and studied chemistry in Marburg before he emigrated to England in 1852. Claus died in London in the year 1900.

Flue gas is the gas exiting to the atmosphere via a flue, which is a pipe or channel for conveying exhaust gases from a fireplace, oven, furnace, boiler or steam generator. Quite often, the flue gas refers to the combustion exhaust gas produced at power plants. Its composition depends on what is being burned, but it will usually consist of mostly nitrogen derived from the combustion of air, carbon dioxide, and water vapor as well as excess oxygen. It further contains a small percentage of a number of pollutants, such as particulate matter, carbon monoxide, nitrogen oxides, and sulfur oxides.

Petroleum coke, abbreviated coke or petcoke, is a final carbon-rich solid material that derives from oil refining, and is one type of the group of fuels referred to as cokes. Petcoke is the coke that, in particular, derives from a final cracking process—a thermo-based chemical engineering process that splits long chain hydrocarbons of petroleum into shorter chains—that takes place in units termed coker units. Stated succinctly, coke is the "carbonization product of high-boiling hydrocarbon fractions obtained in petroleum processing ." Petcoke is also produced in the production of synthetic crude oil (syncrude) from bitumen extracted from Canada’s oil sands and from Venezuela's Orinoco oil sands.
Selective catalytic reduction (SCR) is a means of converting nitrogen oxides, also referred to as NO
x with the aid of a catalyst into diatomic nitrogen, and water. A gaseous reductant, typically anhydrous ammonia, aqueous ammonia or urea, is added to a stream of flue or exhaust gas and is adsorbed onto a catalyst. Carbon dioxide, CO
2 is a reaction product when urea is used as the reductant.

Coal pollution mitigation, often called clean coal, is a series of systems and technologies that seek to mitigate the pollution and other environmental effects normally associated with the burning of coal, which is widely regarded as the dirtiest of the common fuels for industrial processes and power generation.
In atmospheric chemistry, NO
x is a generic term for the nitrogen oxides that are most relevant for air pollution, namely nitric oxide (NO) and nitrogen dioxide. These gases contribute to the formation of smog and acid rain, as well as affecting tropospheric ozone.

Fluid catalytic cracking (FCC) is one of the most important conversion processes used in petroleum refineries. It is widely used to convert the high-boiling, high-molecular weight hydrocarbon fractions of petroleum crude oils into more valuable gasoline, olefinic gases, and other products. Cracking of petroleum hydrocarbons was originally done by thermal cracking, which has been almost completely replaced by catalytic cracking because it produces more gasoline with a higher octane rating. It also produces byproduct gases that have more carbon-carbon double bonds, and hence more economic value, than those produced by thermal cracking.
Hydrodesulfurization (HDS) is a catalytic chemical process widely used to remove sulfur (S) from natural gas and from refined petroleum products, such as gasoline or petrol, jet fuel, kerosene, diesel fuel, and fuel oils. The purpose of removing the sulfur, and creating products such as ultra-low-sulfur diesel, is to reduce the sulfur dioxide emissions that result from using those fuels in automotive vehicles, aircraft, railroad locomotives, ships, gas or oil burning power plants, residential and industrial furnaces, and other forms of fuel combustion.
Ammonia is one of the most highly produced inorganic chemicals. There are numerous large-scale ammonia production plants worldwide, producing a total of 144 million tonnes of nitrogen in 2016. China produced 31.9% of the worldwide production, followed by Russia with 8.7%, India with 7.5%, and the United States with 7.1%. 80% or more of the ammonia produced is used for fertilizing agricultural crops. Ammonia is also used for the production of plastics, fibers, explosives, nitric acid and intermediates for dyes and pharmaceuticals.

A flue-gas stack, also known as a smoke stack, chimney stack or simply as a stack, is a type of chimney, a vertical pipe, channel or similar structure through which combustion product gases called flue gases are exhausted to the outside air. Flue gases are produced when coal, oil, natural gas, wood or any other fuel is combusted in an industrial furnace, a power plant's steam-generating boiler, or other large combustion device. Flue gas is usually composed of carbon dioxide (CO2) and water vapor as well as nitrogen and excess oxygen remaining from the intake combustion air. It also contains a small percentage of pollutants such as particulate matter, carbon monoxide, nitrogen oxides and sulfur oxides. The flue gas stacks are often quite tall, up to 400 metres (1300 feet) or more, so as to disperse the exhaust pollutants over a greater area and thereby reduce the concentration of the pollutants to the levels required by governmental environmental policy and environmental regulation.
Desulfurization [Am.] or desulphurisation [Brit.] is a chemical process for the removal of sulfur from a material. This involves either the removal of sulfur from a molecule or the removal of sulfur compounds from a mixture such as oil refinery streams.
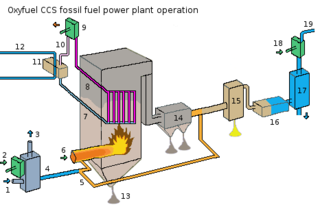
Oxy-fuel combustion is the process of burning a fuel using pure oxygen instead of air as the primary oxidant. Since the nitrogen component of air is not heated, fuel consumption is reduced, and higher flame temperatures are possible. Historically, the primary use of oxy-fuel combustion has been in welding and cutting of metals, especially steel, since oxy-fuel allows for higher flame temperatures than can be achieved with an air-fuel flame.
The wet sulfuric acid process (WSA process) is one of the key gas desulfurization processes on the market today. Since the Danish catalyst company Haldor Topsoe introduced and patented this technology in the late 1980s, it has been recognised as an efficient process for recovering sulfur from various process gasses in the form of commercial quality sulfuric acid (H2SO4), with simultaneous production of high pressure steam. The WSA process is applied in all industries where removal of sulfur is an issue.
The RDF-PowerStation is a peripheral thermal recovery plant, which is based on renewable energy.










