
Flaviviridae is a family of enveloped positive-strand RNA viruses which mainly infect mammals and birds. They are primarily spread through arthropod vectors. The family gets its name from the yellow fever virus; flavus is Latin for "yellow", and yellow fever in turn was named because of its propensity to cause jaundice in victims. There are 89 species in the family divided among four genera. Diseases associated with the group include: hepatitis (hepaciviruses), hemorrhagic syndromes, fatal mucosal disease (pestiviruses), hemorrhagic fever, encephalitis, and the birth defect microcephaly (flaviviruses).

Hepadnaviridae is a family of viruses. Humans, apes, and birds serve as natural hosts. There are currently 18 species in this family, divided among 5 genera. Its best-known member is hepatitis B virus. Diseases associated with this family include: liver infections, such as hepatitis, hepatocellular carcinomas, and cirrhosis. It is the sole accepted family in the order Blubervirales.
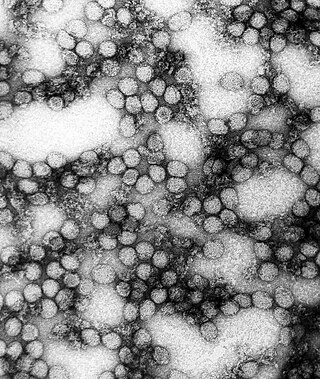
Flavivirus is a genus of positive-strand RNA viruses in the family Flaviviridae. The genus includes the West Nile virus, dengue virus, tick-borne encephalitis virus, yellow fever virus, Zika virus and several other viruses which may cause encephalitis, as well as insect-specific flaviviruses (ISFs) such as cell fusing agent virus (CFAV), Palm Creek virus (PCV), and Parramatta River virus (PaRV). While dual-host flaviviruses can infect vertebrates as well as arthropods, insect-specific flaviviruses are restricted to their competent arthropods. The means by which flaviviruses establish persistent infection in their competent vectors and cause disease in humans depends upon several virus-host interactions, including the intricate interplay between flavivirus-encoded immune antagonists and the host antiviral innate immune effector molecules.
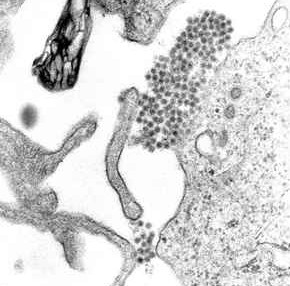
Dengue virus (DENV) is the cause of dengue fever. It is a mosquito-borne, single positive-stranded RNA virus of the family Flaviviridae; genus Flavivirus. Four serotypes of the virus have been found, a reported fifth has yet to be confirmed, all of which can cause the full spectrum of disease. Nevertheless, scientists' understanding of dengue virus may be simplistic as, rather than distinct antigenic groups, a continuum appears to exist. This same study identified 47 strains of dengue virus. Additionally, coinfection with and lack of rapid tests for Zika virus and chikungunya complicate matters in real-world infections.

Rubella virus (RuV) is the pathogenic agent of the disease rubella, transmitted only between humans via the respiratory route, and is the main cause of congenital rubella syndrome when infection occurs during the first weeks of pregnancy.
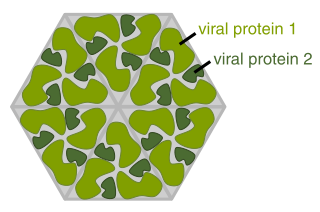
A viral protein is both a component and a product of a virus. Viral proteins are grouped according to their functions, and groups of viral proteins include structural proteins, nonstructural proteins, regulatory proteins, and accessory proteins. Viruses are non-living and do not have the means to reproduce on their own, instead depending on their host cell's resources in order to reproduce. Thus, viruses do not code for many of their own viral proteins, and instead use the host cell's machinery to produce the viral proteins they require for replication.

Tick-borne encephalitis virus (TBEV) is a positive-strand RNA virus associated with tick-borne encephalitis in the genus Flavivirus.
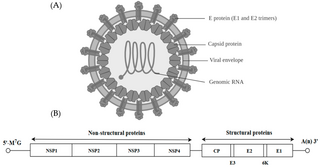
Alphavirus is a genus of RNA viruses, the sole genus in the Togaviridae family. Alphaviruses belong to group IV of the Baltimore classification of viruses, with a positive-sense, single-stranded RNA genome. There are 32 alphaviruses, which infect various vertebrates such as humans, rodents, fish, birds, and larger mammals such as horses, as well as invertebrates. Alphaviruses that could infect both vertebrates and arthropods are referred dual-host alphaviruses, while insect-specific alphaviruses such as Eilat virus and Yada yada virus are restricted to their competent arthropod vector. Transmission between species and individuals occurs mainly via mosquitoes, making the alphaviruses a member of the collection of arboviruses – or arthropod-borne viruses. Alphavirus particles are enveloped, have a 70 nm diameter, tend to be spherical, and have a 40 nm isometric nucleocapsid.

Orbivirus is a genus of double-stranded RNA viruses in the family Reoviridae and subfamily Sedoreovirinae. Unlike other reoviruses, orbiviruses are arboviruses. They can infect and replicate within a wide range of arthropod and vertebrate hosts. Orbiviruses are named after their characteristic doughnut-shaped capsomers.
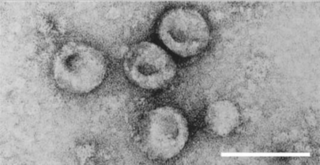
Pestivirus is a genus of viruses, in the family Flaviviridae. Viruses in the genus Pestivirus infect mammals, including members of the family Bovidae and the family Suidae. There are 11 species in this genus. Diseases associated with this genus include: hemorrhagic syndromes, abortion, and fatal mucosal disease.

A viral disease occurs when an organism's body is invaded by pathogenic viruses, and infectious virus particles (virions) attach to and enter susceptible cells.
Batai orthobunyavirus (BATV) is a RNA virus belonging to order Bunyavirales, genus Orthobunyavirus.
Entebbe bat virus is an infectious disease caused by a Flavivirus that is closely related to yellow fever.
Spondweni virus is an arbovirus, or arthropod-borne virus, which is a member of the family Flaviviridae and the genus Flavivirus. It is part of the Spondweni serogroup which consists of the Sponweni virus and the Zika virus (ZIKV). The Spondweni virus was first isolated in Nigeria in 1952, and ever since, SPONV transmission and activity have been reported throughout Africa. Its primary vector of transmission is the sylvatic mosquito Aedes circumluteolus, though it has been isolated from several different types of mosquito. Transmission of the virus into humans can lead to a viral infection known as Spondweni fever, with symptoms ranging from headache and nausea to myalgia and arthralgia. However, SPONV is phylogenetically close to the ZIKV, it is commonly misdiagnosed as ZIKV along with other viral illnesses.
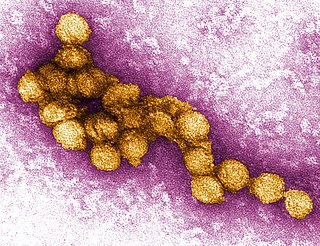
West Nile virus (WNV) is a single-stranded RNA virus that causes West Nile fever. It is a member of the family Flaviviridae, from the genus Flavivirus, which also contains the Zika virus, dengue virus, and yellow fever virus. The virus is primarily transmitted by mosquitoes, mostly species of Culex. The primary hosts of WNV are birds, so that the virus remains within a "bird–mosquito–bird" transmission cycle. The virus is genetically related to the Japanese encephalitis family of viruses.

Middelburg virus (MIDV) is an alphavirus of the Old World Group that has likely endemic and zoonotic potential. It is of the viral family Togaviridae. It was isolated from mosquitos in 1957 in South Africa, MDIV antigens have now been found in livestock, horses, and humans.
Umatilla virus(UMAV) is a dsRNA virus in the family Reoviridae, subfamily Sedoreovirinae, and the genus Orbivirus. This arbovirus was first isolated in 1969 in Umatilla County, Oregon in a group of Culex pipiens mosquitoes. The viral host is the Passer domesticus bird with the vectors being Culex mosquitoes.
Yokose virus (YOKV) is in the genus Flavivirus of the family Flaviviridae. Flaviviridae are often found in arthropods, such as mosquitoes and ticks, and may also infect humans. The genus Flavivirus includes over 50 known viruses, including Yellow Fever, West Nile Virus, Zika Virus, and Japanese Encephalitis. Yokose virus is a new member of the Flavivirus family that has only been identified in a few bat species. Bats have been associated with several emerging zoonotic diseases such as Ebola and SARS.

Modoc virus (MODV) is a rodent-associated flavivirus. Small and enveloped, MODV contains positive single-stranded RNA. Taxonomically, MODV is part of the Flavivirus genus and Flaviviridae family. The Flavivirus genus includes nearly 80 viruses, both vector-borne and no known vector (NKV) species. Known flavivirus vector-borne viruses include Dengue virus, Yellow Fever virus, tick-borne encephalitis virus, and West Nile virus.
Rio Negro virus is an alphavirus that was first isolated in Argentina in 1980. The virus was first called Ag80-663 but was renamed to Rio Negro virus in 2005. It is a former member of the Venezuelan equine encephalitis complex (VEEC), which are a group of alphaviruses in the Americas that have the potential to emerge and cause disease. Río Negro virus was recently reclassified as a distinct species. Closely related viruses include Mucambo virus and Everglades virus.















