Related Research Articles
An oncolytic virus is a virus that preferentially infects and kills cancer cells. As the infected cancer cells are destroyed by oncolysis, they release new infectious virus particles or virions to help destroy the remaining tumour. Oncolytic viruses are thought not only to cause direct destruction of the tumour cells, but also to stimulate host anti-tumour immune system responses. Oncolytic viruses also have the ability to affect the tumor micro-environment in multiple ways.

Regeneron Pharmaceuticals, Inc. is an American biotechnology company headquartered in Westchester County, New York. The company was founded in 1988. Originally focused on neurotrophic factors and their regenerative capabilities, giving rise to its name, the company then branched out into the study of both cytokine and tyrosine kinase receptors, which gave rise to their first product, which is a VEGF-trap.
Aflibercept, sold under the brand names Eylea among others, is a medication used to treat wet macular degeneration and metastatic colorectal cancer. It was developed by Regeneron Pharmaceuticals and is approved in the United States and the European Union.
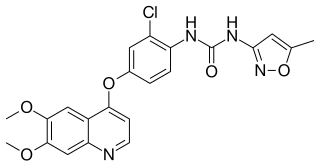
Tivozanib, sold under the brand name Fotivda, is a medication used for the treatment of advanced renal cell carcinoma. It is an oral VEGF receptor tyrosine kinase inhibitor.
Oncolytics Biotech Inc. is a Canadian company headquartered in Calgary, Alberta, that is developing an intravenously delivered immuno-oncolytic virus called pelareorep for the treatment of solid tumors and hematological malignancies. Pelareorep is a non-pathogenic, proprietary isolate of the unmodified reovirus that: induces selective tumor lysis and promotes an inflamed tumor phenotype through innate and adaptive immune responses.
Pelareorep is a proprietary isolate of the unmodified human reovirus being developed as a systemically administered immuno-oncological viral agent for the treatment of solid tumors and hematological malignancies. Pelareorep is an oncolytic virus, which means that it preferentially lyses cancer cells. Pelareorep also promotes an inflamed tumor phenotype through innate and adaptive immune responses. Preliminary clinical trials indicate that it may have anti-cancer effects across a variety of cancer types when administered alone and in combination with other cancer therapies.
JX-594 is an oncolytic virus is designed to target and destroy cancer cells. It is also known as Pexa-Vec, INN pexastimogene devacirepvec) and was constructed in Dr. Edmund Lattime's lab at Thomas Jefferson University, tested in clinical trials on melanoma patients, and licensed and further developed by SillaJen.
Onyx Pharmaceuticals Inc. has been a biopharmaceutical company headquartered in South San Francisco, California. The company developed and marketed medicines for the treatment of cancer. Onyx was founded in 1992 by Kevin J. Kinsella and Frank McCormick Ph.D., FRS. McCormick served as the chief scientific officer until 1998, while Kinsella was the firm's chairman. In 2009, the company acquired Proteolix, Inc., a private biotechnology company, for $276 million in cash plus additional milestone payments. In January 2012, the company was named "the top biotechnology takeover target in 2012" through an industry survey. Onyx president and chief executive officer (CEO) Tony Coles had said that Onyx liked its prospects as an independent company and was focused on bringing new therapies to patients. However, at the end of August 2013, Amgen announced it was acquiring Onyx in an agreed $10.4 billion deal.

Brivanib alaninate (INN/USAN) also known as BMS-582664 is an investigational, anti-tumorigenic drug for oral administration. The drug is being developed by Bristol-Myers Squibb for the treatment of hepatocellular carcinoma or HCC, the most common type of liver cancer. Brivanib is no longer in active development.
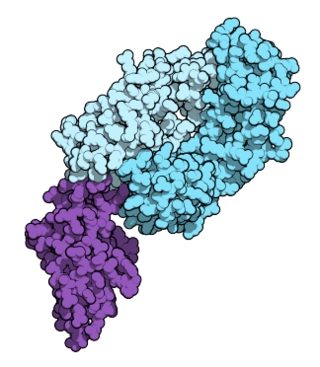
Nivolumab, sold under the brand name Opdivo, is an anti-cancer medication used to treat a number of types of cancer. This includes melanoma, lung cancer, malignant pleural mesothelioma, renal cell carcinoma, Hodgkin lymphoma, head and neck cancer, urothelial carcinoma, colon cancer, esophageal squamous cell carcinoma, liver cancer, gastric cancer, and esophageal or gastroesophageal junction cancer. It is administered intravenously.

Jennerex Biotherapeutics, Inc. was an American private biopharmaceutical company that developed the oncolytic viruses JX-594 and JX-929 among others. By creating oncolytic viruses that can (1) kill tumor cells directly through lysis, (2) activate the immune system by delivering genes that encode immunostimulants and by overcoming tumor cell-induced immunological tolerance, and (3) reduce tumor nutrient supply through the destruction of blood vessels, Jennerex aimed to create a novel approach to treating and possibly curing cancer.

Talimogene laherparepvec, sold under the brand name Imlygic, is a biopharmaceutical medication used to treat melanoma that cannot be operated on; it is injected directly into a subset of lesions which generates a systemic immune response against the recipient's cancer. The final four year analysis from the pivotal phase 3 study upon which TVEC was approved by the FDA showed a 31.5% response rate with a 16.9% complete response (CR) rate. There was also a substantial and statistically significant survival benefit in patients with earlier metastatic disease and in patients who hadn't received prior systemic treatment for melanoma. The earlier stage group had a reduction in the risk of death of approximately 50% with one in four patients appearing to have met, or be close to be reaching, the medical definition of cure. Real world use of talimogene laherparepvec have shown response rates of up to 88.5% with CR rates of up to 61.5%.

Many variants of herpes simplex virus have been considered for viral therapy of cancer; the early development of these was thoroughly reviewed in the journal Cancer Gene Therapy in 2002. This page describes the most notable variants—those tested in clinical trials: G207, HSV1716, NV1020 and Talimogene laherparepvec. These attenuated versions are constructed by deleting viral genes required for infecting or replicating inside normal cells but not cancer cells, such as ICP34.5, ICP6/UL39, and ICP47.

Bavarian Nordic A/S is a fully integrated biotechnology company focused on the development, manufacturing and commercialization of vaccines for infectious diseases and cancer immunotherapies. The company is headquartered in Hellerup, Denmark, with a manufacturing facility in Kvistgård, and an additional site in Hørsholm. The company has a research and development facility in Martinsried, Germany, and offices in Zug, Switzerland, and Morrisville, North Carolina. The company uses viral vectors in its research and development.
Varlilumab is a monoclonal antibody designed for immunotherapy for solid tumors and hematologic malignancies. It is an anti-CD27 antibody and helps activate T-cells.
GL-ONC1 is an investigational therapeutic product consisting of the clinical grade formulation of the laboratory strain GLV-1h68, an oncolytic virus developed by Genelux Corporation. GL-ONC1 is currently under evaluation in Phase I/II human clinical trials in the United States and Europe.
Genelux Corporation is a late clinical-stage public company developing a pipeline of next-generation oncolytic viral immunotherapies for patients suffering from aggressive and/or difficult-to-treat solid tumor types. The Company’s most advanced product candidate, Olvi-Vec, is a proprietary, modified strain of the vaccinia virus (VACV), a stable DNA virus with a large engineering capacity.
Viralytics Ltd is an Australian biotechnology company working in the field of oncolytic viruses, that is, viruses that preferentially infect and kill cancer cells. The company's oncolytic virus product, called Cavatak, is currently in clinical trials in metastatic melanoma and other cancers. The drug was granted Orphan Drug status in advanced melanoma in December 2005.
Cemiplimab, sold under the brand name Libtayo, is a monoclonal antibody medication for the treatment of squamous cell skin cancer. Cemiplimab belongs to a class of drugs that binds to the programmed death receptor-1 (PD-1), blocking the PD-1/PD-L1 pathway.
Transgene S.A. is a French biotechnology company founded in 1979. It is based in Illkirch-Graffenstaden, near Strasbourg, and develops and manufactures immunotherapies for the treatment of cancer.
References
- 1 2 INTERNET, CENTUM. "SOLVE® > TECHNOLOGY > Sillajen". www.sillajen.com (in Korean). Retrieved 2018-06-17.
- ↑ INTERNET, CENTUM. "JX-900 Series > PIPELINE > Sillajen". www.sillajen.com (in Korean). Retrieved 2018-06-17.
- ↑ Zeh, Herbert J; Downs-Canner, Stephanie; McCart, J Andrea; Guo, Zong Sheng; Rao, Uma N M; Ramalingam, Lekshmi; Thorne, Stephen H; Jones, Heather L; Kalinski, Pawel (2015-01-01). "First-in-man Study of Western Reserve Strain Oncolytic Vaccinia Virus: Safety, Systemic Spread, and Antitumor Activity". Molecular Therapy. 23 (1): 202–214. doi:10.1038/mt.2014.194. ISSN 1525-0016. PMC 4426804 . PMID 25292189.
- ↑ INTERNET, CENTUM. "Development Pipeline > PIPELINE > Sillajen". www.sillajen.com.
- ↑ "Hepatocellular Carcinoma Study Comparing Vaccinia Virus Based Immunotherapy Plus Sorafenib vs Sorafenib Alone - Full Text View - ClinicalTrials.gov" . Retrieved 2018-06-16.
- ↑ "A Study of Metronomic CP and JX-594 in Patients With Advanced Breast Cancer and Advanced Soft-tissue Sarcoma (METROmaJX) - Full Text View - ClinicalTrials.gov" . Retrieved 2018-06-16.
- ↑ "A Trial to Evaluate the Safety and Efficacy of the Combination of the Oncolytic Immunotherapy Pexa-Vec With the PD-1 Receptor Blocking Antibody Nivolumab in the First-line Treatment of Advanced Hepatocellular Carcinoma (HCC) - Full Text View - ClinicalTrials.gov" . Retrieved 2018-06-16.
- ↑ "Immunization Strategy With Intra-tumoral Injections of Pexa-Vec With Ipilimumab in Metastatic / Advanced Solid Tumors. - Full Text View - ClinicalTrials.gov" . Retrieved 2018-06-16.
- ↑ "Pexa-Vec Partnerships". www.sillajen.com. Archived from the original on 2017-11-04. Retrieved 2018-06-16.
- ↑ Inc., Regeneron Pharmaceuticals. "Regeneron and SillaJen Announce Immuno-Oncology Clinical Study Agreement for Combination Treatment in Kidney Cancer". www.prnewswire.com. Retrieved 2018-06-16.
{{cite web}}:|last=has generic name (help) - ↑ "Jennerex Biotherapeutics Inc". BioCentury – BCIQ. Retrieved 2018-06-17.
- ↑ "SillaJen Announces Agreement to Acquire Jennerex, Inc" . Retrieved 2018-06-17.
- ↑ Inc., SillaJen. "SillaJen Announces Agreement with the FDA for a Special Protocol Assessment for Upcoming Phase 3 Pexa-Vec Trial in Advanced Liver Cancer". www.prnewswire.com. Retrieved 2018-06-17.
{{cite web}}:|last=has generic name (help) - ↑ "글로벌첨단바이오의약품". 글로벌첨단바이오의약품.
- ↑ "215600:KOSDAQ Stock Quote - SillaJen Inc". Bloomberg.com. Retrieved 2018-06-17.
- ↑ "Sillajen shares dip below IPO price on market debut" . Retrieved 2018-06-17.
- ↑ "Regeneron and SillaJen Announce Immuno-Oncology Clinical Study Agreement for Combination Treatment in Kidney Cancer (NASDAQ:REGN)". investor.regeneron.com. Retrieved 2018-06-17.
- ↑ Inc., SillaJen. "SillaJen and Lee's Pharmaceutical Announce Approval by the China CFDA to Commence Phase 3 Clinical Trial for Oncolytic Immunotherapy, Pexa-Vec, in Liver Cancer". www.prnewswire.com. Retrieved 2018-06-17.
{{cite web}}:|last=has generic name (help) - ↑ Inc., SillaJen. "SillaJen Announces Collaboration with the National Cancer Institute for Development of Combination Therapy with SillaJen's pexastimogene devacirepvec (Pexa-Vec) Oncolytic Immunotherapy". www.prnewswire.com. Retrieved 2018-06-17.
{{cite web}}:|last=has generic name (help)