
Polyvinyl chloride (alternatively: poly(vinyl chloride), colloquial: vinyl or polyvinyl; abbreviated: PVC) is the world's third-most widely produced synthetic polymer of plastic (after polyethylene and polypropylene). About 40 million tons of PVC are produced each year.

Steric effects arise from the spatial arrangement of atoms. When atoms come close together there is generally a rise in the energy of the molecule. Steric effects are nonbonding interactions that influence the shape (conformation) and reactivity of ions and molecules. Steric effects complement electronic effects, which dictate the shape and reactivity of molecules. Steric repulsive forces between overlapping electron clouds result in structured groupings of molecules stabilized by the way that opposites attract and like charges repel.

Photodegradation is the alteration of materials by light. Commonly, the term is used loosely to refer to the combined action of sunlight and air, which cause oxidation and hydrolysis. Often photodegradation is intentionally avoided, since it destroys paintings and other artifacts. It is, however, partly responsible for remineralization of biomass and is used intentionally in some disinfection technologies. Photodegradation does not apply to how materials may be aged or degraded via infrared light or heat, but does include degradation in all of the ultraviolet light wavebands.

2,4-Dimethyl-6-tert-butylphenol is the organic compound with the formula Me2(tert-Bu)C6H2OH (Me = methyl, tert-Bu = tertiary butyl). It is a colorless oil that is classified as an alkylated phenol.
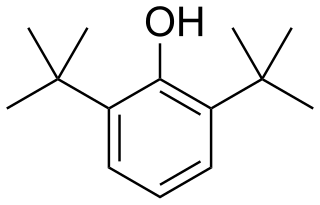
2,6-Di-tert-butylphenol is an organic compound with the structural formula 2,6-((CH3)3C)2C6H3OH. This colorless solid alkylated phenol and its derivatives are used industrially as UV stabilizers and antioxidants for hydrocarbon-based products ranging from petrochemicals to plastics. Illustrative of its usefulness, it prevents gumming in aviation fuels.

Dipotassium phosphate (K2HPO4) (also dipotassium hydrogen orthophosphate; potassium phosphate dibasic) is the inorganic compound with the formula K2HPO4.(H2O)x (x = 0, 3, 6). Together with monopotassium phosphate (KH2PO4.(H2O)x), it is often used as a fertilizer, food additive, and buffering agent. It is a white or colorless solid that is soluble in water.

A release agent is a chemical used to prevent other materials from bonding to surfaces. Release agents aid in processes involving mold release, die-cast release, plastic release, adhesive release, and tire and web release. Release agents are one of many additives used in the production of plastics.

Hindered amine light stabilizers (HALS) are chemical compounds containing an amine functional group that are used as stabilizers in plastics and polymers. These compounds are typically derivatives of tetramethylpiperidine and are primarily used to protect the polymers from the effects of photo-oxidation; as opposed to other forms of polymer degradation such as ozonolysis. They are also increasingly being used as thermal stabilizers, particularly for low and moderate level of heat, however during the high temperature processing of polymers they remain less effective than traditional phenolic antioxidants.

Calcium stearate is a carboxylate salt of calcium, classified as a calcium soap. The salt is a component of some lubricants, surfactants, as well as many foodstuffs. It is a white waxy powder.

Filler materials are particles added to resin or binders that can improve specific properties, make the product cheaper, or a mixture of both. The two largest segments for filler material use is elastomers and plastics. Worldwide, more than 53 million tons of fillers are used every year in application areas such as paper, plastics, rubber, paints, coatings, adhesives, and sealants. As such, fillers, produced by more than 700 companies, rank among the world's major raw materials and are contained in a variety of goods for daily consumer needs. The top filler materials used are ground calcium carbonate (GCC), precipitated calcium carbonate (PCC), kaolin, talc, and carbon black.

4,4′-Dihydroxybenzophenone is an organic compound with the formula (HOC6H4)2CO. This off-white solid is a precursor to, or a degradation product of, diverse commercial materials. It is a potential endocrine disruptor.
Polymer stabilizers are chemical additives which may be added to polymeric materials to inhibit or retard their degradation. Mainly they protect plastic and rubber products against heat, oxidation, and UV light. The biggest quantity of stabilizers is used for polyvinyl chloride (PVC), as the production and processing of this type of plastic would not be possible without stablizing chemicals. Common polymer degradation processes include oxidation, UV-damage, thermal degradation, ozonolysis, combinations thereof such as photo-oxidation, as well as reactions with catalyst residues, dyes, or impurities. All of these degrade the polymer at a chemical level, via chain scission, uncontrolled recombination and cross-linking, which adversely affects many key properties such as strength, malleability, appearance and colour.

An antiozonant, also known as anti-ozonant, is an organic compound that prevents or retards damage caused by ozone. The most important antiozonants are those which prevent degradation of elastomers like rubber. A number of research projects study the application of another type of antiozonants to protect plants as well as salmonids that are affected by the chemicals.
In organic chemistry, transalkylation is a chemical reaction involving the transfer of an alkyl group from one organic compound to another. The reaction is used for the transfer of methyl and ethyl groups between benzene rings. This is of particular value in the petrochemical industry to manufacture p-xylene, styrene, and other aromatic compounds. Motivation for using transalkylation reactions is based on a difference in production and demand for benzene, toluene, and xylenes. Transalkylation can convert toluene, which is overproduced, into benzene and xylene, which are under-produced. Zeolites are often used as catalysts in transalkylation reactions.

A metal salen complex is a coordination compound between a metal cation and a ligand derived from N,N′-bis(salicylidene)ethylenediamine, commonly called salen. The classical example is salcomine, the complex with divalent cobalt Co2+, usually denoted as Co(salen). These complexes are widely investigated as catalysts and enzyme mimics.
A metallic soap is a metallic salt of a fatty acid. Theoretically, soaps can be made of any metal, although not all enjoy practical uses. Varying the metal can strongly affect the properties of the compound, particularly its solubility.
Basic lead phosphite is an inorganic compound with the proposed composition Pb3O(OH)2(HPO3). The compound contains the phosphite anion, which provides the reducing properties associated with the application of this material.

Tris(2,4-di-tert-butylphenyl)phosphite is an organophosphorus compound with the formula [(C4H9)2C6H3O]3P. This white solid is a widely used stabilizer in polymers where it functions as an antioxidant as well as other roles. The compound is a phosphite ester derived from 2,4-di-tert-butylphenol.

Salpn is the common name for a chelating ligand, properly called N,N′-bis(salicylidene)-1,2-propanediamine, used as a motor oil additive.

4-tert-Butylphenol is an organic compound with the formula (CH3)3CC6H4OH. It is one of three isomeric tert-butyl phenols. It is a white solid with a distinct phenolic odor. It dissolves in basic water.


















