
Erich Armand Arthur Joseph Hückel was a German physicist and physical chemist. He is known for two major contributions:
The terpenoids, also known as isoprenoids, are a class of naturally occurring organic chemicals derived from the 5-carbon compound isoprene and its derivatives called terpenes, diterpenes, etc. While sometimes used interchangeably with "terpenes", terpenoids contain additional functional groups, usually containing oxygen. When combined with the hydrocarbon terpenes, terpenoids comprise about 80,000 compounds. They are the largest class of plant secondary metabolites, representing about 60% of known natural products. Many terpenoids have substantial pharmacological bioactivity and are therefore of interest to medicinal chemists.

Terpenes are a class of natural products consisting of compounds with the formula (C5H8)n for n ≥ 2. Terpenes are major biosynthetic building blocks. Comprising more than 30,000 compounds, these unsaturated hydrocarbons are produced predominantly by plants, particularly conifers. In plants, terpenes and terpenoids are important mediators of ecological interactions, while some insects use some terpenes as a form of defense. Other functions of terpenoids include cell growth modulation and plant elongation, light harvesting and photoprotection, and membrane permeability and fluidity control.

Colchicine is a medication used to prevent and treat gout, to treat familial Mediterranean fever and Behçet's disease, and to reduce the risk of myocardial infarction. The American College of Rheumatology recommends colchicine, nonsteroidal anti-inflammatory drugs (NSAIDs) or steroids in the treatment of gout. Other uses for colchicine include the management of pericarditis.

Cupressaceae or the cypress family is a family of conifers. The family includes 27–30 genera, which include the junipers and redwoods, with about 130–140 species in total. They are monoecious, subdioecious or (rarely) dioecious trees and shrubs up to 116 m (381 ft) tall. The bark of mature trees is commonly orange- to red-brown and of stringy texture, often flaking or peeling in vertical strips, but smooth, scaly or hard and square-cracked in some species.

Michael James Steuart Dewar was an American theoretical chemist.

Tropone or 2,4,6-cycloheptatrien-1-one is an organic compound with some importance in organic chemistry as a non-benzenoid aromatic. The compound consists of a ring of seven carbon atoms with three conjugated alkene groups and a ketone group. The related compound tropolone (2-hydroxy-2,4,6-cycloheptatrien-1-one) has an additional alcohol group next to the ketone. Tropones are uncommon in natural products, with the notable exception of the 2-hydroxyl derivatives, which are called tropolones.

Tropolone is an organic compound with the chemical formula C7H5(OH)O. It is a pale yellow solid that is soluble in organic solvents. The compound has been of interest to research chemists because of its unusual electronic structure and its role as a ligand precursor. Although not usually prepared from tropone, it can be viewed as its derivative with a hydroxyl group in the 2-position.
Polyphenol oxidase, an enzyme involved in fruit browning, is a tetramer that contains four atoms of copper per molecule.

Dopamine beta-hydroxylase (DBH), also known as dopamine beta-monooxygenase, is an enzyme that in humans is encoded by the DBH gene. Dopamine beta-hydroxylase catalyzes the conversion of dopamine to norepinephrine.
The enzyme stipitatonate decarboxylase (EC 4.1.1.60) catalyzes the chemical reaction

Ramial chipped wood (RCW), also called BRF, is a type of woodchips made solely from small to medium-sized branches. The adjective "ramial" refers to branches (rami). RCW is a forest product used in agriculture for mulching and soil enrichment. It may be laid on top of the soil, mixed into it, or composted first and then applied.

Thujaplicin is any of three isomeric tropolone-related natural products that have been isolated from the softwoods of the trees of Cupressaceae family. These compounds are known for their antibacterial, antifungal, and antioxidant properties. They were the first natural tropolones to be made synthetically.

Hinokitiol (β-thujaplicin) is a natural monoterpenoid found in the wood of trees in the family Cupressaceae. It is a tropolone derivative and one of the thujaplicins. Hinokitiol is used in oral and skin care products, and is a food additive used in Japan.
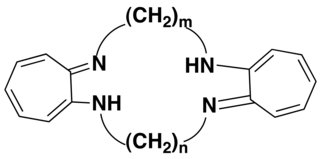
The tropocoronand ligand (H2TC-m,n) is a macrocyclic ligand in which two aminotroponiminate rings are connected to one another via polymethylene linker chains of length m and n. Double deprotonation of the ligand yields a dianionic macrocylic species that is capable of binding divalent transition metal ions to form neutral complexes [M(TC-m,n)]. The 2-aminotroponeimine units are bridged by polymethylene linker chains with all four nitrogen atoms of the tropocoronand ligand bonded to a metal atom.
Malettinins are polyketide-derived antimicrobial compounds produced by fungal ascomycetes in the genus Hypoxylon.

Roy Michael Harrison is a British environmental scientist. He has been Queen Elizabeth II Birmingham Centenary Professor of Environmental Health at the University of Birmingham since 1991, and is a distinguished adjunct professor at King Abdulaziz University in Jeddah, Saudi Arabia.
Prof Peter Ludwig Israel Pauson FRSE FRIC (1925–2013) was a German–Jewish emigrant who settled in Britain and who is remembered for his contributions to chemistry, most notably the Pauson–Khand reaction and as joint discoverer of ferrocene.

Tetsuo Nozoe was a Japanese organic chemist. He is known for the discovery of hinokitiol, a seven-membered aromatic compound, and studying non-benzenoid aromatic compounds.

Thujaplicinol is either of two isomeric tropolone-related natural products. They are found in tree species primarily in bark, needles, xylem, of the family of Cupressaceae like the Cupressus, Thuja, Juniperus and Thujopsis. The thujaplicinols are structurally equivalent to the thujaplicins with an additional hydroxyl group. They belong to the class of natural terpenoids having two free hydroxyl groups at C3 and C5 position.















