
Rhabdoviridae is a family of negative-strand RNA viruses in the order Mononegavirales. Vertebrates, invertebrates, plants, fungi and protozoans serve as natural hosts. Diseases associated with member viruses include rabies encephalitis caused by the rabies virus, and flu-like symptoms in humans caused by vesiculoviruses. The name is derived from Ancient Greek rhabdos, meaning rod, referring to the shape of the viral particles. The family has 40 genera, most assigned to three subfamilies.
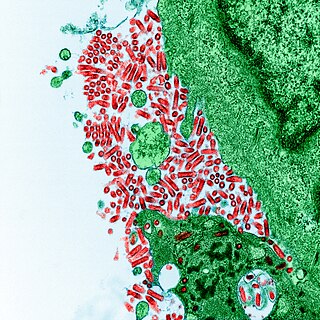
Rabies virus, scientific name Rabies lyssavirus, is a neurotropic virus that causes rabies in animals, including humans. Rabies transmission can occur through the saliva of animals and less commonly through contact with human saliva. Rabies lyssavirus, like many rhabdoviruses, has an extremely wide host range. In the wild it has been found infecting many mammalian species, while in the laboratory it has been found that birds can be infected, as well as cell cultures from mammals, birds, reptiles and insects. Rabies is reported in more than 150 countries and on all continents except Antarctica. The main burden of disease is reported in Asia and Africa, but some cases have been reported also in Europe in the past 10 years, especially in returning travellers.

Bunyavirales is an order of segmented negative-strand RNA viruses with mainly tripartite genomes. Member viruses infect arthropods, plants, protozoans, and vertebrates. It is the only order in the class Ellioviricetes. The name Bunyavirales derives from Bunyamwera, where the original type species Bunyamwera orthobunyavirus was first discovered. Ellioviricetes is named in honor of late virologist Richard M. Elliott for his early work on bunyaviruses.
Chandipura vesiculovirus (CHPV) is a member of the Rhabdoviridae family that is associated with an encephalitic illness in humans. It was first identified in 1965 after isolation from the blood of two patients from Chandipura village in Maharashtra state, India and has been associated with a number of otherwise unexplained outbreaks of encephalitic illness in central India. The most recent occurred in Andhra Pradesh and Maharashtra in June–August 2003 with 329 children affected and 183 deaths. Further sporadic cases and deaths in children were observed in Gujarat state in 2004.

Baculoviridae is a family of viruses. Arthropods, among the most studied being Lepidoptera, Hymenoptera and Diptera, serve as natural hosts. Currently, 85 species are placed in this family, assigned to four genera.
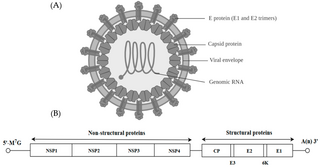
Alphavirus is a genus of RNA viruses, the sole genus in the Togaviridae family. Alphaviruses belong to group IV of the Baltimore classification of viruses, with a positive-sense, single-stranded RNA genome. There are 32 alphaviruses, which infect various vertebrates such as humans, rodents, fish, birds, and larger mammals such as horses, as well as invertebrates. Alphaviruses that could infect both vertebrates and arthropods are referred dual-host alphaviruses, while insect-specific alphaviruses such as Eilat virus and Yada yada virus are restricted to their competent arthropod vector. Transmission between species and individuals occurs mainly via mosquitoes, making the alphaviruses a member of the collection of arboviruses – or arthropod-borne viruses. Alphavirus particles are enveloped, have a 70 nm diameter, tend to be spherical, and have a 40 nm isometric nucleocapsid.

Potyvirus is a genus of positive-strand RNA viruses in the family Potyviridae. Plants serve as natural hosts. Like begomoviruses, members of this genus may cause significant losses in agricultural, pastoral, horticultural, and ornamental crops. More than 200 species of aphids spread potyviruses, and most are from the subfamily Aphidinae. The genus contains 190 species and potyviruses account for about thirty percent of all currently known plant viruses.

Orthobunyavirus is a genus of the Peribunyaviridae family in the order Bunyavirales. There are currently ~170 viruses recognised in this genus. These have been assembled into 103 species and 20 serogroups.
Potato virus Y (PVY) is a plant pathogenic virus of the family Potyviridae, and one of the most important plant viruses affecting potato production.

Strawberry vein banding virus (SVBV) is a plant pathogenic virus and a member of the family Caulimoviridae.
Mason-Pfizer monkey virus (M-PMV), formerly Simian retrovirus (SRV), is a species of retroviruses that usually infect and cause a fatal immune deficiency in Asian macaques. The ssRNA virus appears sporadically in mammary carcinoma of captive macaques at breeding facilities which expected as the natural host, but the prevalence of this virus in feral macaques remains unknown. M-PMV was transmitted naturally by virus-containing body fluids, via biting, scratching, grooming, and fighting. Cross contaminated instruments or equipment (fomite) can also spread this virus among animals.
Orchid fleck dichorhavirus, commonly called Orchid fleck virus (OFV), is a non-enveloped, segmented, single-stranded (ss) RNA negative-strand virus, transmitted by the false spider mite, Brevipalpus californicus. OFV causes necrotic and chlorotic lesions on the leaves of many genera in the family Orchidaceae.
Batai orthobunyavirus (BATV) is a RNA virus belonging to order Bunyavirales, genus Orthobunyavirus.
Lily virus X (LVX) is a pathogenic ssRNA(+) plant virus of the family Alphaflexiviridae and the order Tymovirales.
Entebbe bat virus is an infectious disease caused by a Flavivirus that is closely related to yellow fever.

Avian metaavulavirus 2, formerly Avian paramyxovirus 2, is a species of virus belonging to the family Paramyxoviridae and genus Metaavulavirus. The virus is a negative strand RNA virus containing a monopartite genome. Avian metaavulavirus 2 is one of nine species belonging to the genus Metaavulavirus. The most common serotype of Avulavirinae is serotype 1, the cause of Newcastle disease (ND). Avian metaavulavirus 2 has been known to cause disease, specifically mild respiratory infections in domestic poultry, including turkeys and chickens, and has many economic effects on egg production and poultry industries. The virus was first isolated from a strain in Yucaipa, California in 1956. Since then, other isolates of the virus have been isolated worldwide.
West Caucasian bat lyssavirus (WCBL) is a member of genus Lyssavirus, family Rhabdoviridae and order Mononegavirales. This virus was first isolated from Miniopterus schreibersii, in the western Caucasus Mountains of southeastern Europe in 2002. WCBL is the most divergent form of Lyssavirus, and is found in Miniopterus bats (insectivorous), Rousettus aegyptiacus, and Eidolon helvum. The latter two are both fruit bats. The virus is fragile as it can be inactivated by UV light and chemicals, such as ether, chloroform, and bleach. WCBL has not been known to infect humans thus far.
Bat mumps orthorubulavirus, formerly Bat mumps rubulavirus (BMV), is a member of genus Orthorubulavirus, family Paramyxoviridae, and order Mononegavirales. Paramyxoviridae viruses were first isolated from bats using heminested PCR with degenerate primers. This process was then followed by Sanger sequencing. A specific location of this virus is not known because it was isolated from bats worldwide. Although multiple paramyxoviridae viruses have been isolated worldwide, BMV specifically has not been isolated thus far. However, BMV was detected in African fruit bats, but no infectious form has been isolated to date. It is known that BMV is transmitted through saliva in the respiratory system of bats. While the virus was considered its own species for a few years, phylogenetic analysis has since shown that it is a member of Mumps orthorubulavirus.
Rio Negro virus is an alphavirus that was first isolated in Argentina in 1980. The virus was first called Ag80-663 but was renamed to Rio Negro virus in 2005. It is a former member of the Venezuelan equine encephalitis complex (VEEC), which are a group of alphaviruses in the Americas that have the potential to emerge and cause disease. Río Negro virus was recently reclassified as a distinct species. Closely related viruses include Mucambo virus and Everglades virus.










