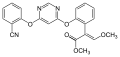
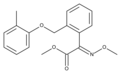
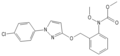
Natural strobilurins
Strobilurin A
Strobilurin A (also known as mucidin) is produced by Oudemansiella mucida , Strobilurus tenacellus , Bolinea lutea , and others. [6] [7] [8] When first isolated it was incorrectly assigned as the E E E geometric isomer but was later identified by total synthesis as being the E Z E isomer, as shown. [5] : 694
9-Methoxystrobilurin A
9-Methoxystrobilurin A is produced by Favolaschia spp. [7]
Strobilurin B
Strobilurin B is produced by S. tenacellus. [7]
Strobilurin C
Strobilurin C is produced by X. longipes and X. melanotricha . [7] [8]
Strobilurin D and G
Strobilurin D is produced by Cyphellopsis anomala . [8] Its structure was originally incorrectly assigned and is now considered to be identical to that of strobilurin G, produced by B. lutea. [7] [8] A related material, hydroxystrobilurin D, with an additional hydroxyl group attached to the methyl of the main chain is produced by Mycena sanguinolenta . [7]
Strobilurin E
Strobilurin E is produced by Crepidotus fulvotomentosus [8] and Favolaschia spp. [7]
Strobilurin F2
Strobilurin F2 is produced by B. lutea. [6]
Strobilurin H
Strobilurin H is produced by B. lutea. [7] The natural product with a phenolic hydroxy group in place of the aromatic methoxy group of strobilurin H is called strobilurin F1 and is found in C. anomala [8] and Agaricus spp. [6]
Strobilurin X
Oudemansins
The oudemansins are closely related to the strobilurins and are also quinone outside inhibitors. [7]
Oudemansin A with R1 = R2 = H was first described in 1979, after being isolated from mycelial fermentations of the basidiomycete fungus Oudemansiella mucida. [9] Later it was found in cultures of the basidiomycete fungi Mycena polygramma and Xerula melanotricha. The latter fungus also produces oudemansin B, with R1 = MeO and R2 = Cl. Oudemansin X, with R1 = H and R2 = MeO was isolated from Oudemansiella radicata . [6]