In organic chemistry, a ketone is an organic compound with the structure R−C(=O)−R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group −C(=O)−. The simplest ketone is acetone, with the formula (CH3)2CO. Many ketones are of great importance in biology and in industry. Examples include many sugars (ketoses), many steroids, and the solvent acetone.
Monosaccharides, also called simple sugars, are the simplest forms of sugar and the most basic units (monomers) from which all carbohydrates are built. Simply, this is the structural unit of carbohydrates.

In organic chemistry, an aldehyde is an organic compound containing a functional group with the structure R−CH=O. The functional group itself can be referred to as an aldehyde but can also be classified as a formyl group. Aldehydes are a common motif in many chemicals important in technology and biology.

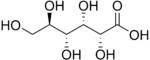
In chemistry, a hexose is a monosaccharide (simple sugar) with six carbon atoms. The chemical formula for all hexoses is C6H12O6, and their molecular weight is 180.156 g/mol.
Benedict's reagent is a chemical reagent and complex mixture of sodium carbonate, sodium citrate, and copper(II) sulfate pentahydrate. It is often used in place of Fehling's solution to detect the presence of reducing sugars. The presence of other reducing substances also gives a positive result. Such tests that use this reagent are called the Benedict's tests. A positive test with Benedict's reagent is shown by a color change from clear blue to brick-red with a precipitate.
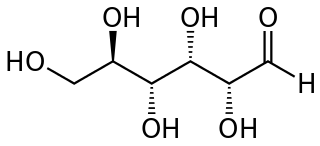
An aldose is a monosaccharide with a carbon backbone chain with a carbonyl group on the endmost carbon atom, making it an aldehyde, and hydroxyl groups connected to all the other carbon atoms. Aldoses can be distinguished from ketoses, which have the carbonyl group away from the end of the molecule, and are therefore ketones.

In organic chemistry, Fehling's solution is a chemical reagent used to differentiate between water-soluble carbohydrate and ketone functional groups, and as a test for reducing sugars and non-reducing sugars, supplementary to the Tollens' reagent test. The test was developed by German chemist Hermann von Fehling in 1849.
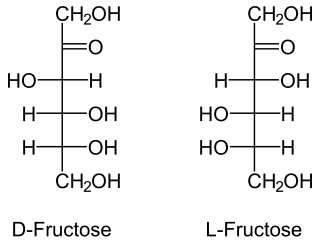
In organic chemistry, a ketose is a monosaccharide containing one ketone group per molecule. The simplest ketose is dihydroxyacetone, which has only three carbon atoms. It is the only ketose with no optical activity. All monosaccharide ketoses are reducing sugars, because they can tautomerize into aldoses via an enediol intermediate, and the resulting aldehyde group can be oxidised, for example in the Tollens' test or Benedict's test. Ketoses that are bound into glycosides, for example in the case of the fructose moiety of sucrose, are nonreducing sugars.
A reducing sugar is any sugar that is capable of acting as a reducing agent. In an alkaline solution, a reducing sugar forms some aldehyde or ketone, which allows it to act as a reducing agent, for example in Benedict's reagent. In such a reaction, the sugar becomes a carboxylic acid.
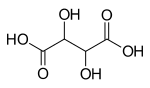
Aldaric acids are a group of sugar acids, where the terminal hydroxyl and carbonyl groups of the sugars have been replaced by terminal carboxylic acids, and are characterised by the formula HO2C-(CHOH)n-CO2H. Oxidation of just the aldehyde yields an aldonic acid while oxidation of just the terminal hydroxyl group yields an uronic acid.) Aldaric acids cannot form cyclic hemiacetals like unoxidized sugars, but they can sometimes form lactones.

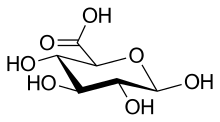
Glucuronic acid is a uronic acid that was first isolated from urine. It is found in many gums such as gum arabic, xanthan, and kombucha tea and is important for the metabolism of microorganisms, plants and animals.

Transketolase is an enzyme that, in humans, is encoded by the TKT gene. It participates in both the pentose phosphate pathway in all organisms and the Calvin cycle of photosynthesis. Transketolase catalyzes two important reactions, which operate in opposite directions in these two pathways. In the first reaction of the non-oxidative pentose phosphate pathway, the cofactor thiamine diphosphate accepts a 2-carbon fragment from a 5-carbon ketose (D-xylulose-5-P), then transfers this fragment to a 5-carbon aldose (D-ribose-5-P) to form a 7-carbon ketose (sedoheptulose-7-P). The abstraction of two carbons from D-xylulose-5-P yields the 3-carbon aldose glyceraldehyde-3-P. In the Calvin cycle, transketolase catalyzes the reverse reaction, the conversion of sedoheptulose-7-P and glyceraldehyde-3-P to pentoses, the aldose D-ribose-5-P and the ketose D-xylulose-5-P.
The Kiliani–Fischer synthesis, named for German chemists Heinrich Kiliani and Emil Fischer, is a method for synthesizing monosaccharides. It proceeds via synthesis and hydrolysis of a cyanohydrin, followed by reduction of the intermediate acid to the aldehyde, thus elongating the carbon chain of an aldose by one carbon atom while preserving stereochemistry on all the previously present chiral carbons. The new chiral carbon is produced with both stereochemistries, so the product of a Kiliani–Fischer synthesis is a mixture of two diastereomeric sugars, called epimers. For example, D-arabinose is converted to a mixture of D-glucose and D-mannose.

Uronic acids or alduronic acids are a class of sugar acids with both carbonyl and carboxylic acid functional groups. They are sugars in which the hydroxyl group furthest from the carbonyl group has been oxidized to a carboxylic acid. Usually the sugar is an aldose, but fructuronic acid also occurs. Oxidation of the terminal aldehyde instead yields an aldonic acid, while oxidation of both the terminal hydroxyl group and the aldehyde yields an aldaric acid. The names of uronic acids are generally based on their parent sugars, for example, the uronic acid analog of glucose is glucuronic acid. Uronic acids derived from hexoses are known as hexuronic acids and uronic acids derived from pentoses are known as penturonic acids.

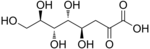
An aldonic acid is any of a family of sugar acids obtained by oxidation of the aldehyde functional group of an aldose to form a carboxylic acid functional group. Thus, their general chemical formula is HO2C(CHOH)nCH2OH. Oxidation of the terminal hydroxyl group instead of the terminal aldehyde yields a uronic acid, while oxidation of both terminal ends yields an aldaric acid.

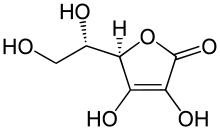
Neuraminic acid (5-amino-3,5-dideoxy-D-glycero-D-galacto-non-2-ulosonic acid) is an acidic (in particular ulosonic) amino sugar with a backbone formed by nine carbon atoms. Although 9-carbon sugars do not occur naturally, neuraminic acid may be regarded as a theoretical 9-carbon ketose in which the first link of the chain (the –CH2OH at position 1) is oxidised into a carboxyl group (–C(=O)OH), the hydroxyl group at position 3 is deoxidised (oxygen is removed from it), and the hydroxyl group at position 5 is substituted with an amino group (–NH2). Neuraminic acid may also be visualized as the product of an aldol-condensation of pyruvic acid and D-mannosamine (2-amino-2-deoxy-mannose).
In carbohydrate chemistry, the Lobry de Bruyn–Van Ekenstein transformation also known as the Lobry de Bruyn–Alberda van Ekenstein transformation is the base or acid catalyzed transformation of an aldose into the ketose isomer or vice versa, with a tautomeric enediol as reaction intermediate. Ketoses may be transformed into 3-ketoses, etcetera. The enediol is also an intermediate for the epimerization of an aldose or ketose.
The Amadori rearrangement is an organic reaction describing the acid or base catalyzed isomerization or rearrangement reaction of the N-glycoside of an aldose or the glycosylamine to the corresponding 1-amino-1-deoxy-ketose. The reaction is important in carbohydrate chemistry, specifically the glycation of hemoglobin.
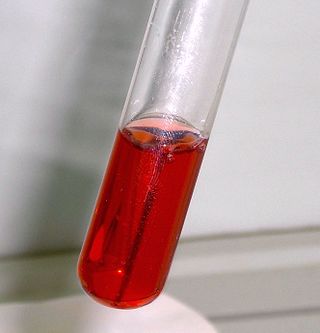
Seliwanoff’s test is a chemical test which distinguishes between aldose and ketose sugars. If the sugar contains a ketone group, it is a ketose. If a sugar contains an aldehyde group, it is an aldose. This test relies on the principle that, when heated, ketoses are more rapidly dehydrated than aldoses. It is named after Theodor Seliwanoff, the chemist who devised the test. When added to a solution containing ketoses, a red color is formed rapidly indicating a positive test. When added to a solution containing aldoses, a slower forming light pink is observed instead.
Monosaccharide nomenclature is the naming system of the building blocks of carbohydrates, the monosaccharides, which may be monomers or part of a larger polymer. Monosaccharides are subunits that cannot be further hydrolysed in to simpler units. Depending on the number of carbon atom they are further classified into trioses, tetroses, pentoses, hexoses etc., which is further classified in to aldoses and ketoses depending on the type of functional group present in them.