Chemical thermodynamics is the study of the interrelation of heat and work with chemical reactions or with physical changes of state within the confines of the laws of thermodynamics. Chemical thermodynamics involves not only laboratory measurements of various thermodynamic properties, but also the application of mathematical methods to the study of chemical questions and the spontaneity of processes.

Enthalpy is the sum of a thermodynamic system's internal energy and the product of its pressure and volume. It is a state function in thermodynamics used in many measurements in chemical, biological, and physical systems at a constant external pressure, which is conveniently provided by the large ambient atmosphere. The pressure–volume term expresses the work that was done against constant external pressure to establish the system's physical dimensions from to some final volume , i.e. to make room for it by displacing its surroundings. The pressure-volume term is very small for solids and liquids at common conditions, and fairly small for gases. Therefore, enthalpy is a stand-in for energy in chemical systems; bond, lattice, solvation, and other chemical "energies" are actually enthalpy differences. As a state function, enthalpy depends only on the final configuration of internal energy, pressure, and volume, not on the path taken to achieve it.

Salinity is the saltiness or amount of salt dissolved in a body of water, called saline water. It is usually measured in g/L or g/kg.
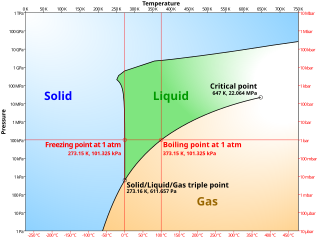
A phase diagram in physical chemistry, engineering, mineralogy, and materials science is a type of chart used to show conditions at which thermodynamically distinct phases occur and coexist at equilibrium.

In chemistry, solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form such a solution.

In thermodynamics, the Gibbs free energy is a thermodynamic potential that can be used to calculate the maximum amount of work, other than pressure-volume work, that may be performed by a thermodynamically closed system at constant temperature and pressure. It also provides a necessary condition for processes such as chemical reactions that may occur under these conditions. The Gibbs free energy is expressed asWhere:
The standard state of a material is a reference point used to calculate its properties under different conditions. A degree sign (°) or a superscript Plimsoll symbol (⦵) is used to designate a thermodynamic quantity in the standard state, such as change in enthalpy (ΔH°), change in entropy (ΔS°), or change in Gibbs free energy (ΔG°). The degree symbol has become widespread, although the Plimsoll is recommended in standards, see discussion about typesetting below.

In the thermodynamics of equilibrium, a state function, function of state, or point function for a thermodynamic system is a mathematical function relating several state variables or state quantities that depend only on the current equilibrium thermodynamic state of the system, not the path which the system has taken to reach that state. A state function describes equilibrium states of a system, thus also describing the type of system. A state variable is typically a state function so the determination of other state variable values at an equilibrium state also determines the value of the state variable as the state function at that state. The ideal gas law is a good example. In this law, one state variable is a function of other state variables so is regarded as a state function. A state function could also describe the number of a certain type of atoms or molecules in a gaseous, liquid, or solid form in a heterogeneous or homogeneous mixture, or the amount of energy required to create such a system or change the system into a different equilibrium state.
Exergy, often referred to as "available energy" or "useful work potential", is a fundamental concept in the field of thermodynamics and engineering. It plays a crucial role in understanding and quantifying the quality of energy within a system and its potential to perform useful work. Exergy analysis has widespread applications in various fields, including energy engineering, environmental science, and industrial processes.

Thermodynamics is expressed by a mathematical framework of thermodynamic equations which relate various thermodynamic quantities and physical properties measured in a laboratory or production process. Thermodynamics is based on a fundamental set of postulates, that became the laws of thermodynamics.
The potential temperature of a parcel of fluid at pressure is the temperature that the parcel would attain if adiabatically brought to a standard reference pressure , usually 1,000 hPa (1,000 mb). The potential temperature is denoted and, for a gas well-approximated as ideal, is given by

The thermodynamic properties of materials are intensive thermodynamic parameters which are specific to a given material. Each is directly related to a second order differential of a thermodynamic potential. Examples for a simple 1-component system are:
In thermodynamics, a departure function is defined for any thermodynamic property as the difference between the property as computed for an ideal gas and the property of the species as it exists in the real world, for a specified temperature T and pressure P. Common departure functions include those for enthalpy, entropy, and internal energy.

Thermodynamic databases contain information about thermodynamic properties for substances, the most important being enthalpy, entropy, and Gibbs free energy. Numerical values of these thermodynamic properties are collected as tables or are calculated from thermodynamic datafiles. Data is expressed as temperature-dependent values for one mole of substance at the standard pressure of 101.325 kPa, or 100 kPa. Both of these definitions for the standard condition for pressure are in use.

Ocean heat content (OHC) or ocean heat uptake (OHU) is the energy absorbed and stored by oceans. To calculate the ocean heat content, it is necessary to measure ocean temperature at many different locations and depths. Integrating the areal density of a change in enthalpic energy over an ocean basin or entire ocean gives the total ocean heat uptake. Between 1971 and 2018, the rise in ocean heat content accounted for over 90% of Earth's excess energy from global heating. The main driver of this increase was anthropogenic forcing via rising greenhouse gas emissions. By 2020, about one third of the added energy had propagated to depths below 700 meters.
Spice, spiciness, or spicity, symbol τ, is a term in oceanography referring to variations in the temperature and salinity of seawater over space or time, whose combined effects leave the water's density unchanged. For a given spice, any change in temperature is offset by a change in salinity to maintain unchanged density. An increase in temperature decreases density, but an increase in salinity increases density. Such density-compensated thermohaline variability is ubiquitous in the upper ocean. Warmer, saltier water is more spicy while cooler, less salty water is more minty. For a density ratio of 1, all the thermohaline variability is spice, and there are no density fluctuations.
Conservative temperature is a thermodynamic property of seawater. It is derived from the potential enthalpy and is recommended under the TEOS-10 standard as a replacement for potential temperature as it more accurately represents the heat content in the ocean.

Trevor John McDougallFAGU is a physical oceanographer specialising in ocean mixing and the thermodynamics of seawater. He is Emeritus Scientia Professor of Ocean Physics in the School of Mathematics and Statistics at the University of New South Wales, Sydney, Australia, and is Past President of the International Association for the Physical Sciences of the Oceans (IAPSO) of the International Union of Geodesy and Geophysics.

The Turner angleTu, introduced by Ruddick(1983) and named after J. Stewart Turner, is a parameter used to describe the local stability of an inviscid water column as it undergoes double-diffusive convection. The temperature and salinity attributes, which generally determine the water density, both respond to the water vertical structure. By putting these two variables in orthogonal coordinates, the angle with the axis can indicate the importance of the two in stability. Turner angle is defined as:
The Haline contraction coefficient, abbreviated as β, is a coefficient that describes the change in ocean density due to a salinity change, while the potential temperature and the pressure are kept constant. It is a parameter in the Equation Of State (EOS) of the ocean. β is also described as the saline contraction coefficient and is measured in [kg]/[g] in the EOS that describes the ocean. An example is TEOS-10. This is the thermodynamic equation of state.







