Related Research Articles

The endoplasmic reticulum (ER) is a type of organelle found in eukaryotic cells that forms an interconnected network of flattened, membrane-enclosed sacs or tube-like structures known as cisternae. The membranes of the ER are continuous with the outer nuclear membrane. The endoplasmic reticulum occurs in most eukaryotic cells, but is absent from red blood cells and spermatozoa.

The endomembrane system is composed of the different membranes that are suspended in the cytoplasm within a eukaryotic cell. These membranes divide the cell into functional and structural compartments, or organelles. In eukaryotes the organelles of the endomembrane system include: the nuclear membrane, the endoplasmic reticulum, the Golgi apparatus, lysosomes, vesicles, endosomes, and plasma (cell) membrane among others. The system is defined more accurately as the set of membranes that form a single functional and developmental unit, either being connected directly, or exchanging material through vesicle transport. Importantly, the endomembrane system does not include the membranes of chloroplasts or mitochondria, but might have evolved from the latter.

The Golgi apparatus, also known as the Golgi complex, Golgi body, or simply the Golgi, is an organelle found in most eukaryotic cells. Part of the endomembrane system in the cytoplasm, the Golgi apparatus packages proteins into membrane-bound vesicles inside the cell before the vesicles are sent to their destination. The Golgi apparatus resides at the intersection of the secretory, lysosomal, and endocytic pathways. It is of particular importance in processing proteins for secretion, containing a set of glycosylation enzymes that attach various sugar monomers to proteins as the proteins move through the apparatus.
Protein targeting or protein sorting is the biological mechanism by which proteins are transported to their appropriate destinations in the cell or outside it. Proteins can be targeted to the inner space of an organelle, different intracellular membranes, plasma membrane, or to exterior of the cell via secretion. This delivery process is carried out based on information contained in the protein itself. Correct sorting is crucial for the cell; errors can lead to diseases.

In cell biology, a vesicle is a structure within or outside a cell, consisting of liquid or cytoplasm enclosed by a lipid bilayer. Vesicles form naturally during the processes of secretion (exocytosis), uptake (endocytosis) and transport of materials within the plasma membrane. Alternatively, they may be prepared artificially, in which case they are called liposomes. If there is only one phospholipid bilayer, they are called unilamellar liposome vesicles; otherwise they are called multilamellar. The membrane enclosing the vesicle is also a lamellar phase, similar to that of the plasma membrane, and intracellular vesicles can fuse with the plasma membrane to release their contents outside the cell. Vesicles can also fuse with other organelles within the cell. A vesicle released from the cell is known as an extracellular vesicle.

COPII is a coatomer, a type of vesicle coat protein that transports proteins from the rough endoplasmic reticulum to the Golgi apparatus. This process is termed anterograde transport, in contrast to the retrograde transport associated with the COPI protein. The name "COPII" refers to the specific coat protein complex that initiates the budding process. The coat consists of large protein subcomplexes that are made of four different protein subunits.

COPI is a coatomer, a protein complex that coats vesicles transporting proteins from the cis end of the Golgi complex back to the rough endoplasmic reticulum (ER), where they were originally synthesized, and between Golgi compartments. This type of transport is termed as retrograde transport, in contrast to the anterograde transport associated with the COPII protein. The name "COPI" refers to the specific coat protein complex that initiates the budding process on the cis-Golgi membrane. The coat consists of large protein subcomplexes that are made of seven different protein subunits, namely α, β, β', γ, δ, ε and ζ.

SAR1A or Sar1 is a protein involved in membrane trafficking. It is a monomeric small GTPase found in COPII vesicles. It regulates the assembly and disassembly of COPII coats.
The exocyst is an octameric protein complex involved in vesicle trafficking, specifically the tethering and spatial targeting of post-Golgi vesicles to the plasma membrane prior to vesicle fusion. It is implicated in a number of cell processes, including exocytosis, cell migration, and growth.
A secretory protein is any protein, whether it be endocrine or exocrine, which is secreted by a cell. Secretory proteins include many hormones, enzymes, toxins, and antimicrobial peptides. Secretory proteins are synthesized in the endoplasmic reticulum.
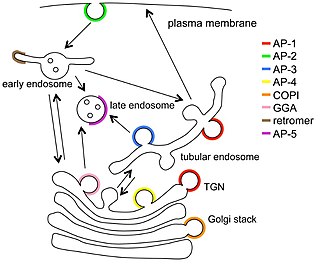
Vesicular transport adaptor proteins are proteins involved in forming complexes that function in the trafficking of molecules from one subcellular location to another. These complexes concentrate the correct cargo molecules in vesicles that bud or extrude off of one organelle and travel to another location, where the cargo is delivered. While some of the details of how these adaptor proteins achieve their trafficking specificity has been worked out, there is still much to be learned.
The coatomer is a protein complex that coats membrane-bound transport vesicles. Two types of coatomers are known:

General vesicular transport factor p115 is a protein that in humans is encoded by the USO1 gene.

Syntaxin-5 is a protein that in humans is encoded by the STX5 gene.

Trafficking protein particle complex subunit 3 is a protein that in humans is encoded by the TRAPPC3 gene.
Sec23 homolog A , also known as SEC23A, is a protein which in humans is encoded by the SEC23A gene.

Protein transport protein Sec61 subunit alpha isoform 1 is a protein that in humans is encoded by the SEC61A1 gene.

Cranio-lenticulo-sutural dysplasia is a neonatal/infancy disease caused by a disorder in the 14th chromosome. It is an autosomal recessive disorder, meaning that both recessive genes must be inherited from each parent in order for the disease to manifest itself. The disease causes a significant dilation of the endoplasmic reticulum in fibroblasts of the host with CLSD. Due to the distension of the endoplasmic reticulum, export of proteins from the cell is disrupted.
Unconventional protein secretion represents a manner in which the proteins are delivered to the surface of plasma membrane or extracellular matrix independent of the Endoplasmic reticulum or Golgi apparatus. This includes cytokines and mitogens with crucial function in complex processes such as inflammatory response or tumor-induced angiogenesis. Most of these proteins are involved in processes in higher eukaryotes, however an unconventional export mechanism was found in lower eukaryotes too. Even proteins folded in their correct conformation can pass plasma membrane this way, unlike proteins transported via ER/Golgi pathway. Two types of unconventional protein secretion are these: signal-peptid-containing proteins and cytoplasmatic and nuclear proteins that are missing an ER-signal peptide (1).
Rab GTPases are molecular switches that regulate membrane traffic. They are active in their GTP-bound form and inactive when bound to GDP. The GTPase YPT1, and its mammalian homologue Rab1, regulate membrane-tethering events on three different pathways: autophagy, ER-Golgi, and intra-Golgi traffic. In the yeast Saccharomyces cerevisiae, many of the ATG proteins needed for macroautophagy are shared with the biosynthetic cytoplasm to the vacuole-targeting (CVT) pathway that transports certain hydrolases into the vacuole. Both pathways require YPT1; however, only the macroautophagy pathway is conserved in higher eukaryotes. In the macroautophagy pathway, Rab1 mediates the recruitment of Atg1 to the PAS. Rab1 regulates macroautophagy by recruiting its effector, Atg1, to the PAS to tether Atg9 vesicles to each other or to other membranes.
References
- ↑ Cai et al.,
- ↑ Van Bergen NJ, Guo Y, Al-Deri N, Lipatova Z, Stanga D, Zhao S, Murtazina R, Gyurkovska V, Pehlivan D, Mitani T, Gezdirici A, Antony J, Collins F, Willis MJH, Coban Akdemir ZH, Liu P, Punetha J, Hunter JV, Jhangiani SN, Fatih JM, Rosenfeld JA, Posey JE, Gibbs RA, Karaca E, Massey S, Ranasinghe TG, Sleiman P, Troedson C, Lupski JR, Sacher M, Segev N, Hakonarson H, Christodoulou J (2019) Deficiencies in vesicular transport mediated by TRAPPC4 are associated with severe syndromic intellectual disability. Brain