Thermogenin is a mitochondrial carrier protein found in brown adipose tissue (BAT). It is used to generate heat by non-shivering thermogenesis, and makes a quantitatively important contribution to countering heat loss in babies which would otherwise occur due to their high surface area-volume ratio.

2,4-Dinitrophenol (2,4-DNP or simply DNP) is an organic compound with the formula HOC6H3(NO2)2. It has been used in explosives manufacturing and as a pesticide and herbicide.
Thermogenic means tending to produce heat, and the term is commonly applied to drugs which increase heat through metabolic stimulation, or to microorganisms which create heat within organic waste. Approximately all enzymatic reaction in the human body is thermogenic, which gives rise to the basal metabolic rate.

A nucleophilic aromatic substitution is a substitution reaction in organic chemistry in which the nucleophile displaces a good leaving group, such as a halide, on an aromatic ring. Aromatic rings are usually nucleophilic, but some aromatic compounds do undergo nucleophilic substitution. Just as normally nucleophilic alkenes can be made to undergo conjugate substitution if they carry electron-withdrawing substituents, so normally nucleophilic aromatic rings also become electrophilic if they have the right substituents.
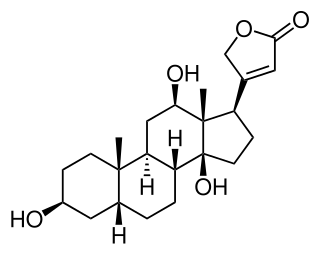
Digoxigenin (DIG) is a steroid found exclusively in the flowers and leaves of the plants Digitalis purpurea, Digitalis orientalis and Digitalis lanata (foxgloves), where it is attached to sugars, to form the glycosides.

2,4 Dienoyl-CoA reductase also known as DECR1 is an enzyme which in humans is encoded by the DECR1 gene which resides on chromosome 8. This enzyme catalyzes the following reactions

An uncoupling protein (UCP) is a mitochondrial inner membrane protein that is a regulated proton channel or transporter. An uncoupling protein is thus capable of dissipating the proton gradient generated by NADH-powered pumping of protons from the mitochondrial matrix to the mitochondrial intermembrane space. The energy lost in dissipating the proton gradient via UCPs is not used to do biochemical work. Instead, heat is generated. This is what links UCP to thermogenesis. However, not every type of UCPs are related to thermogenesis. Although UCP2 and UCP3 are closely related to UCP1, UCP2 and UCP3 do not affect thermoregulatory abilities of vertebrates. UCPs are positioned in the same membrane as the ATP synthase, which is also a proton channel. The two proteins thus work in parallel with one generating heat and the other generating ATP from ADP and inorganic phosphate, the last step in oxidative phosphorylation. Mitochondria respiration is coupled to ATP synthesis, but is regulated by UCPs. UCPs belong to the mitochondrial carrier (SLC25) family.

Mitochondrial uncoupling protein 3 is a protein that in humans is encoded by the UCP3 gene. The gene is located in chromosome (11q13.4) with an exon count of 7 and is expressed on the inner mitochondrial membrane. Uncoupling proteins transfer anions from the inner mitochondrial membrane to the outer mitochondrial membrane, thereby separating oxidative phosphorylation from synthesis of ATP, and dissipating energy stored in the mitochondrial membrane potential as heat. Uncoupling proteins also reduce generation of reactive oxygen species.

In enzymology, a kynurenine-oxoglutarate transaminase is an enzyme that catalyzes the chemical reaction
A protonophore, also known as a proton translocator, is an ionophore that moves protons across lipid bilayers or other type of membranes. This would otherwise not occur as protons cations (H+) have positive charge and hydrophilic properties, making them unable to cross without a channel or transporter in the form of a protonophore. Protonophores are generally aromatic compounds with a negative charge, that are both hydrophobic and capable of distributing the negative charge over a number of atoms by π-orbitals which delocalize a proton's charge when it attaches to the molecule. Both the neutral and the charged protonophore can diffuse across the lipid bilayer by passive diffusion and simultaneously facilitate proton transport. Protonophores uncouple oxidative phosphorylation via a decrease in the membrane potential of the inner membrane of mitochondria. They stimulate mitochondria respiration and heat production. Protonophores (uncouplers) are often used in biochemistry research to help explore the bioenergetics of chemiosmotic and other membrane transport processes. It has been reported that the protonophore has antibacterial activity by perturbing bacterial proton motive force.
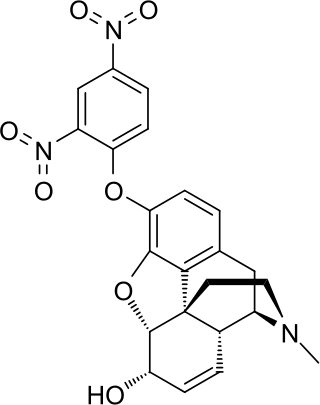
2,4-Dinitrophenylmorphine is an analog of morphine in which a hydroxyl group is substituted with a dinitro phenoxy group.

Dinoseb is a common industry name for 6-sec-butyl-2,4-dinitrophenol, a herbicide in the dinitrophenol family. It is a crystalline orange solid which does not readily dissolve in water. Dinoseb is banned as an herbicide in the European Union (EU) and the United States because of its toxicity.
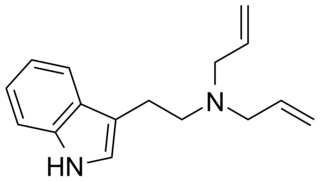
N,N-Diallyltryptamine (DALT) is a tryptamine derivative which has been identified as a new psychoactive substance. It has been used as an intermediate in the preparation of radiolabeled diethyltryptamine.
An uncoupler or uncoupling agent is a molecule that disrupts oxidative phosphorylation in prokaryotes and mitochondria or photophosphorylation in chloroplasts and cyanobacteria by dissociating the reactions of ATP synthesis from the electron transport chain. The result is that the cell or mitochondrion expends energy to generate a proton-motive force, but the proton-motive force is dissipated before the ATP synthase can recapture this energy and use it to make ATP. Uncouplers are capable of transporting protons through mitochondrial and lipid membranes.

Rottlerin (mallotoxin) is a polyphenol natural product isolated from the Asian tree Mallotus philippensis. Rottlerin displays a complex spectrum of pharmacology.

N-Arachidonylglycine (NAGly) is a carboxylic metabolite of the endocannabinoid anandamide (AEA). Since it was first synthesized in 1996, NAGly has been a primary focus of the relatively contemporary field of lipidomics due to its wide range of signaling targets in the brain, the immune system and throughout various other bodily systems. In combination with 2‐arachidonoyl glycerol (2‐AG), NAGly has enabled the identification of a family of lipids often referred to as endocannabinoids. Recently, NAGly has been found to bind to G-protein coupled receptor 18 (GPR18), the putative abnormal cannabidiol receptor. NaGly is an endogenous inhibitor of fatty acid amide hydrolase (FAAH) and thereby increases the ethanolamide endocannabinoids AEA, oleoylethanolamide (OEA) and palmitoylethanolamide (PEA) levels. NaGly is found throughout the body and research on its explicit functions is ongoing.
A mode of toxic action is a common set of physiological and behavioral signs that characterize a type of adverse biological response. A mode of action should not be confused with mechanism of action, which refer to the biochemical processes underlying a given mode of action. Modes of toxic action are important, widely used tools in ecotoxicology and aquatic toxicology because they classify toxicants or pollutants according to their type of toxic action. There are two major types of modes of toxic action: non-specific acting toxicants and specific acting toxicants. Non-specific acting toxicants are those that produce narcosis, while specific acting toxicants are those that are non-narcotic and that produce a specific action at a specific target site.
Fish acute toxicity syndrome (FATS) is a set of common chemical and functional responses in fish resulting from a short-term, acute exposure to a lethal concentration of a toxicant, a chemical or material that can produce an unfavorable effect in a living organism. By definition, modes of action are characterized by FATS because the combination of common responses that represent each fish acute toxicity syndrome characterize an adverse biological effect. Therefore, toxicants that have the same mode of action elicit similar sets of responses in the organism and can be classified by the same fish acute toxicity syndrome.
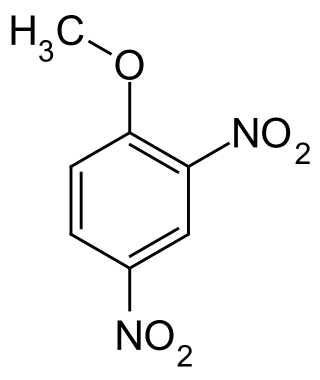
2,4-Dinitroanisole (DNAN) is a low sensitivity organic compound. It has an anisole (methoxybenzene) core, with two nitro groups (–NO2) attached.
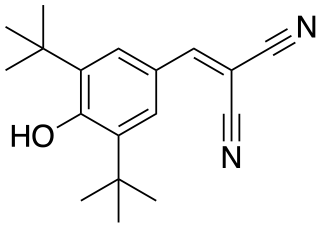
SF-6847 is an uncoupling agent/protonophore. As of 1974 when it was discovered, it was considered most powerful, with a potency over 1800x that of 2,4-dinitrophenol - the prototypical uncoupling agent, and about 3x the effectiveness of 5-chloro-3-tert-butyl-2'-chloro-4'-nitrosalicylanilide.