Related Research Articles

Pancreatic cancer arises when cells in the pancreas, a glandular organ behind the stomach, begin to multiply out of control and form a mass. These cancerous cells have the ability to invade other parts of the body. A number of types of pancreatic cancer are known.
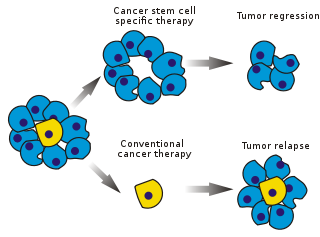
Cancer stem cells (CSCs) are cancer cells that possess characteristics associated with normal stem cells, specifically the ability to give rise to all cell types found in a particular cancer sample. CSCs are therefore tumorigenic (tumor-forming), perhaps in contrast to other non-tumorigenic cancer cells. CSCs may generate tumors through the stem cell processes of self-renewal and differentiation into multiple cell types. Such cells are hypothesized to persist in tumors as a distinct population and cause relapse and metastasis by giving rise to new tumors. Therefore, development of specific therapies targeted at CSCs holds hope for improvement of survival and quality of life of cancer patients, especially for patients with metastatic disease.

Erlotinib, sold under the brand name Tarceva among others, is a medication used to treat non-small cell lung cancer (NSCLC) and pancreatic cancer. Specifically it is used for NSCLC with mutations in the epidermal growth factor receptor (EGFR) — either an exon 19 deletion (del19) or exon 21 (L858R) substitution mutation — which has spread to other parts of the body. It is taken by mouth.
The prolactin receptor (PRLR) is a type I cytokine receptor encoded in humans by the PRLR gene on chromosome 5p13-14. It is the receptor for prolactin (PRL). The PRLR can also bind to and be activated by growth hormone (GH) and human placental lactogen (hPL). The PRLR is expressed in the mammary glands, pituitary gland, and other tissues. It plays an important role in lobuloalveolar development of the mammary glands during pregnancy and in lactation.

Flossie Wong-Staal was a Chinese-American virologist and molecular biologist. She was the first scientist to clone HIV and determine the function of its genes, which was a major step in proving that HIV is the cause of AIDS. From 1990 to 2002, she held the Florence Riford Chair in AIDS Research at the University of California, San Diego (UCSD). She was co-founder and, after retiring from UCSD, she became the chief scientific officer of Immusol, which was renamed iTherX Pharmaceuticals in 2007 when it transitioned to a drug development company focused on hepatitis C and continued as chief scientific officer.

KRAS is a gene that provides instructions for making a protein called K-Ras, a part of the RAS/MAPK pathway. The protein relays signals from outside the cell to the cell's nucleus. These signals instruct the cell to grow and divide (proliferate) or to mature and take on specialized functions (differentiate). It is called KRAS because it was first identified as a viral oncogene in the KirstenRAt Sarcoma virus. The oncogene identified was derived from a cellular genome, so KRAS, when found in a cellular genome, is called a proto-oncogene.

Sanford Burnham Prebys is a 501(c)(3) non-profit medical research institute focusing on basic and translational research, with major research programs in cancer, neurodegeneration, diabetes, infectious, inflammatory, and childhood diseases. The institute also specializes in stem cell research and drug discovery technologies.

Sean J. Morrison is a Canadian-American stem cell biologist and cancer researcher. Morrison is the director of Children's Medical Center Research Institute at UT Southwestern (CRI), a nonprofit research institute established in 2011 as a joint venture between Children’s Health System of Texas and UT Southwestern Medical Center. With Morrison as founding director, CRI was established to perform transformative biomedical research at the interface of stem cell biology, cancer and metabolism to better understand the biological basis of disease. He is a Howard Hughes Medical Institute Investigator, has served as president of the International Society for Stem Cell Research, and is a member of the U.S. National Academy of Medicine, U.S. National Academy of Sciences and European Molecular Biology Organization.

CD47 also known as integrin associated protein (IAP) is a transmembrane protein that in humans is encoded by the CD47 gene. CD47 belongs to the immunoglobulin superfamily and partners with membrane integrins and also binds the ligands thrombospondin-1 (TSP-1) and signal-regulatory protein alpha (SIRPα). CD-47 acts as a don't eat me signal to macrophages of the immune system which has made it a potential therapeutic target in some cancers, and more recently, for the treatment of pulmonary fibrosis.

Tyrosine-protein kinase receptor UFO is an enzyme that in humans is encoded by the AXL gene. The gene was initially designated as UFO, in allusion to the unidentified function of this protein. However, in the years since its discovery, research into AXL's expression profile and mechanism has made it an increasingly attractive target, especially for cancer therapeutics. In recent years, AXL has emerged as a key facilitator of immune escape and drug-resistance by cancer cells, leading to aggressive and metastatic cancers.
Pancreatic stellate cells (PaSCs) are classified as myofibroblast-like cells that are located in exocrine regions of the pancreas. PaSCs are mediated by paracrine and autocrine stimuli and share similarities with the hepatic stellate cell. Pancreatic stellate cell activation and expression of matrix molecules constitute the complex process that induces pancreatic fibrosis. Synthesis, deposition, maturation and remodelling of the fibrous connective tissue can be protective, however when persistent it impedes regular pancreatic function.

Sangeeta N. Bhatia is an American biological engineer and the John J. and Dorothy Wilson Professor at MIT’s Institute for Medical Engineering and Science and Electrical Engineering and Computer Science (EECS) at the Massachusetts Institute of Technology (MIT) in Cambridge, Massachusetts, United States. Bhatia's research investigates applications of micro- and nano-technology for tissue repair and regeneration. She applies ideas from computer technology and engineering to the design of miniaturized biomedical tools for the study and treatment of diseases, in particular liver disease, hepatitis, malaria and cancer.
Jonathan S. Weissman is the Landon T. Clay Professor of Biology at the Massachusetts Institute of Technology, a member of the Whitehead Institute, and a Howard Hughes Medical Institute Investigator. From 1996 to 2020, he was a faculty member in the department of cellular molecular pharmacology at the University of California, San Francisco.

Tessa Laurie Holyoake, was a Scottish haematology-oncology physician. She specialised in chronic myeloid leukaemia (CML), and discovered its stem cell. She was considered a world leading expert in leukaemia research.
JoAnn Trejo is an American pharmacologist, cell biologist, a professor, and also an assistant vice chancellor in the department of health sciences faculty affairs in the Department of Pharmacology at the School of Medicine at University of California, San Diego. She is also the assistant vice chancellor for Health Sciences Faculty Affairs. Trejo studies cell signalling by protease-activated G protein-coupled receptors (GPCRs). She is also actively involved in mentoring, education and outreach activities to increase the diversity of science.
Ruth S. Waterman is an American anesthesiologist, Chair of the Department of Anesthesiology at UC San Diego Health, Associate Clinical Professor at UC San Diego School of Medicine, and Co-Founder and Chief Medical Officer of Commence Bio, a company that specializes in next generation stem cell therapy and cancer immunotherapy. Waterman is known for developing stem cell-based therapies to help patients with pain and advancing methods to personalize pain medicine based on pre-surgery genetic testing.

Dannielle Engle is an American biologist and assistant professor of the regulatory biology laboratory at the Salk Institute for Biological Studies. Engle's research aims at improving detection and treatment of pancreatic cancer.
Donita C. Brady is a cancer biologist and the Presidential Associate Professor of Cancer Biology at the Perelman School of Medicine at the University of Pennsylvania. Her research examines how cells communicate through kinases and nutrient homeostasis, and in particular, the central role of copper and other metals in these interactions.

Relda Marie Cailleau was an American scientist primarily known for her establishment of a series of breast cancer cell lines that have been crucial to the discovery of anticancer drugs and to an understanding of breast cancer biology.

Jaye Cassandra Gardiner is an American cancer researcher at the Fox Chase Cancer Center. Her research considers the microenvironment that surrounds tumors, with a particular focus on pancreatic ductal adenocarcinoma. In 2022, she was the inaugural awardee of the Black in Cancer Postdoctoral Fellowship.
References
- ↑ "Tannishtha Reya | UCSD Profiles". profiles.ucsd.edu. Retrieved 2019-06-26.
- ↑ Reya, Tannishtha; Morrison, Sean J.; Clarke, Michael F.; Weissman, Irving L. (November 2001). "Stem cells, cancer, and cancer stem cells" (PDF). Nature. 414 (6859): 105–111. Bibcode:2001Natur.414..105R. doi:10.1038/35102167. hdl: 2027.42/62862 . ISSN 0028-0836. PMID 11689955. S2CID 4343326.
- ↑ Sedwick, Caitlin (2012-09-03). "Tannishtha Reya: Classic pathways, new views on cancer". The Journal of Cell Biology. 198 (5): 766–767. doi:10.1083/jcb.1985pi. ISSN 0021-9525. PMC 3432759 . PMID 22945930.
- ↑ Buschman, Heather (27 October 2016). "Antibody Breaks Leukemia's Hold, Providing New Therapeutic Approach". UC San Diego Health. Retrieved 2019-06-27.
- ↑ Lytle, Nikki K.; Ferguson, L. Paige; Rajbhandari, Nirakar; Gilroy, Kathryn; Fox, Raymond G.; Deshpande, Anagha; Schürch, Christian M.; Hamilton, Michael; Robertson, Neil (April 2019). "A Multiscale Map of the Stem Cell State in Pancreatic Adenocarcinoma". Cell. 177 (3): 572–586.e22. doi: 10.1016/j.cell.2019.03.010 . PMC 6711371 . PMID 30955884.
- ↑ Sklar, Debbie L. (2019-04-04). "UCSD Researchers Find Inhibiting Hormone Receptor Could Stall Cancer Growth". Times of San Diego. Retrieved 2019-06-27.
- ↑ Sisson, Paul (30 November 2018). "New center aims for pancreatic cancer prevention". medicalxpress.com. Retrieved 2019-06-27.
- ↑ "Editorial Board | Science Signaling". stke.sciencemag.org. Retrieved 2019-06-26.
- ↑ "'Dream Team' Including La Jollans Gets 'Stand Up to Cancer' $7M Grant". Times of San Diego. 2017-10-26. Retrieved 2019-06-26.
- ↑ "Ep 144: "Multiscale Map of Stem Cell State in Pancreatic Adenocarcinoma" Featuring Dr. Tannishtha Reya". Stem Cell Podcast. 2019-06-11. Retrieved 2019-06-26.