| |
| Identifiers | |
|---|---|
3D model (JSmol) | |
| ChemSpider | |
PubChem CID | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C28H28Zr | |
| Molar mass | 455.756 g·mol−1 |
| Appearance | orange solid |
| Density | 1.34-1.39 g/cm3 [1] |
| Melting point | 133–134 °C (271–273 °F; 406–407 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
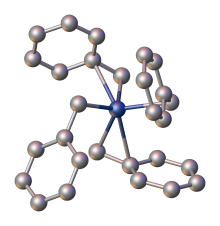
Tetrabenzylzirconium is an organozirconium compound with the formula Zr(CH2C6H5)4. The molecule features diamagnetic Zr(IV) bonded to four benzyl ligands. It is an orange air- and photo-sensitive solid, which is soluble in hydrocarbon solvents. The compound is a precursor to catalysts for the polymerization of olefins. [2] [3]