
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the rest of the molecule's composition. This enables systematic prediction of chemical reactions and behavior of chemical compounds and the design of chemical synthesis. The reactivity of a functional group can be modified by other functional groups nearby. Functional group interconversion can be used in retrosynthetic analysis to plan organic synthesis.

In organic chemistry, aromaticity is a chemical property describing the way in which a conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibits a stabilization stronger than would be expected by the stabilization of conjugation alone. The earliest use of the term was in an article by August Wilhelm Hofmann in 1855. There is no general relationship between aromaticity as a chemical property and the olfactory properties of such compounds.
Cubane is a synthetic hydrocarbon compound with the formula C8H8. It consists of eight carbon atoms arranged at the corners of a cube, with one hydrogen atom attached to each carbon atom. A solid crystalline substance, cubane is one of the Platonic hydrocarbons and a member of the prismanes. It was first synthesized in 1964 by Philip Eaton and Thomas Cole. Before this work, Eaton believed that cubane would be impossible to synthesize due to the "required 90 degree bond angles". The cubic shape requires the carbon atoms to adopt an unusually sharp 90° bonding angle, which would be highly strained as compared to the 109.45° angle of a tetrahedral carbon. Once formed, cubane is quite kinetically stable, due to a lack of readily available decomposition paths. It is the simplest hydrocarbon with octahedral symmetry.
In organic chemistry, a substituent is one or a group of atoms that replaces atoms, thereby becoming a moiety in the resultant (new) molecule.

In chemistry, a molecule or ion is called chiral if it cannot be superposed on its mirror image by any combination of rotations, translations, and some conformational changes. This geometric property is called chirality. The terms are derived from Ancient Greek χείρ (cheir) 'hand'; which is the canonical example of an object with this property.
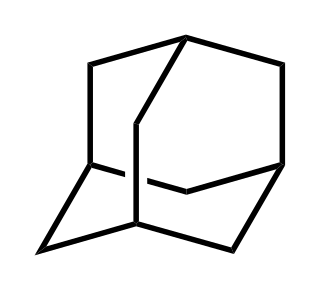
Adamantane is an organic compound with formula C10H16 or, more descriptively, (CH)4(CH2)6. Adamantane molecules can be described as the fusion of three cyclohexane rings. The molecule is both rigid and virtually stress-free. Adamantane is the most stable isomer of C10H16. The spatial arrangement of carbon atoms in the adamantane molecule is the same as in the diamond crystal. This similarity led to the name adamantane, which is derived from the Greek adamantinos (relating to steel or diamond). It is a white solid with a camphor-like odor. It is the simplest diamondoid.
Dodecahedrane is a chemical compound, a hydrocarbon with formula C20H20, whose carbon atoms are arranged as the vertices (corners) of a regular dodecahedron. Each carbon is bound to three neighbouring carbon atoms and to a hydrogen atom. This compound is one of the three possible Platonic hydrocarbons, the other two being cubane and tetrahedrane.

Prismane or 'Ladenburg benzene' is a polycyclic hydrocarbon with the formula C6H6. It is an isomer of benzene, specifically a valence isomer. Prismane is far less stable than benzene. The carbon (and hydrogen) atoms of the prismane molecule are arranged in the shape of a six-atom triangular prism—this compound is the parent and simplest member of the prismanes class of molecules. Albert Ladenburg proposed this structure for the compound now known as benzene. The compound was not synthesized until 1973.

In organic chemistry, a bent bond, also known as a banana bond, is a type of covalent chemical bond with a geometry somewhat reminiscent of a banana. The term itself is a general representation of electron density or configuration resembling a similar "bent" structure within small ring molecules, such as cyclopropane (C3H6) or as a representation of double or triple bonds within a compound that is an alternative to the sigma and pi bond model.
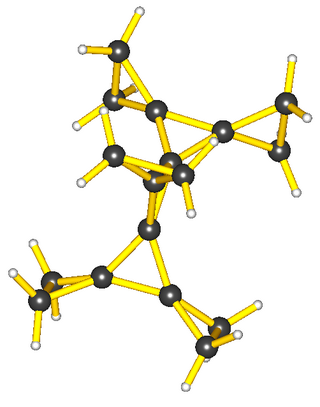
In organic chemistry, spiro compounds are compounds that have at least two molecular rings sharing one common atom. Simple spiro compounds are bicyclic. The presence of only one common atom connecting the two rings distinguishes spiro compounds from other bicyclics. Spiro compounds may be fully carbocyclic or heterocyclic. One common type of spiro compound encountered in educational settings is a heterocyclic one— the acetal formed by reaction of a diol with a cyclic ketone.
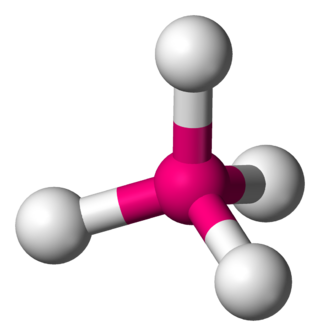
In a tetrahedral molecular geometry, a central atom is located at the center with four substituents that are located at the corners of a tetrahedron. The bond angles are cos−1(−1⁄3) = 109.4712206...° ≈ 109.5° when all four substituents are the same, as in methane as well as its heavier analogues. Methane and other perfectly symmetrical tetrahedral molecules belong to point group Td, but most tetrahedral molecules have lower symmetry. Tetrahedral molecules can be chiral.

Trimethylenemethane is a chemical compound with formula C
4H
6. It is a neutral free molecule with two unsatisfied valence bonds, and is therefore a highly reactive free radical. Formally, it can be viewed as an isobutylene molecule C
4H
8 with two hydrogen atoms removed from the terminal methyl groups.

A rotane is a hydrocarbon consisting of a central cycloalkane ring with cyclopropane units spiro-linked to each corner. The systematic naming pattern for these molecules is "[n]rotane", where n is the number of atoms in the central ring.
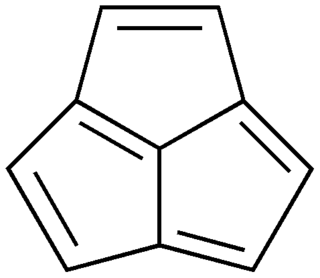
Acepentalene is a tricyclic anti-aromatic compound. Its molecular formula is C10H6. It consists of three five-membered rings fused across three of the five carbon atoms. The central carbon atom in acepentalene is part of all three rings. There are formally five double bonds in acepentalene, so that the molecule formally contains four double bonds on the exterior, and one double bond from the central carbon to the exterior of the ring system.

[1.1.1]Propellane is an organic compound, the simplest member of the propellane family. It is a hydrocarbon with formula C5H6 or C2(CH2)3. The molecular structure consists of three rings of three carbon atoms each, sharing one C–C bond.

[2.2.2]Propellane, formally tricyclo[2.2.2.01,4]octane is an organic compound, a member of the propellane family. It is a hydrocarbon with formula C8H12, or C2(C2H4)3. Its molecule has three rings with four carbon atoms each, sharing one C–C bond.
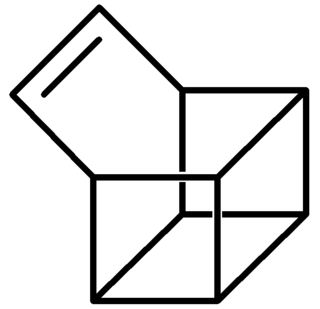
Basketene (IUPAC name: pentacyclo[4.4.0.02,5.03,8.04,7]dec-9-ene) is an organic compound with the formula C10H10. It is a polycyclic alkene and the dehydrogenated version of basketane, which was named for its structural similarity to a basket. Due to its hydrocarbon composition and unique structure, the chemical compound is of considerable interest to those examining energy surfaces of these (CH)10 cage molecules and what possible factors influence their minima. Additionally, the complex structure of this compound has intrigued researchers studying the chemistry of highly strained ring systems. Basketene and its family of derivatives also have important chemical and physical properties. These molecules all tend to have a high standard enthalpy of formation, combined with their high density, leading to possible uses in explosives.

Calicene or triapentafulvalene is a hydrocarbon of the fulvalene class with chemical formula C8H6, composed of a cyclopentadiene ring and a cyclopropene ring linked by a double bond. Its name is derived from the Latin calix meaning "goblet", from its shape.
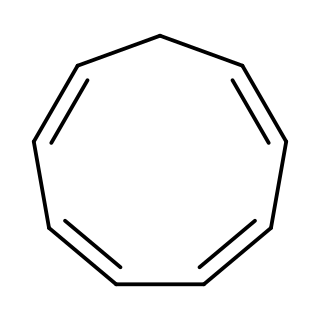
Cyclononatetraene is an organic compound with the formula C9H10. It was first prepared in 1969 by protonation of the corresponding aromatic anion (described below). It is unstable and isomerizes with a half-life of 50 minutes at room temperature to 3a,7a-dihydro-1H-indene via a thermal 6π disrotatory electrocyclic ring closing. Upon exposure to ultraviolet light, it undergoes a photochemical 8π electrocyclic ring closing to give bicyclo[6.1.0]nona-2,4,6-triene.

A triangulane is a hydrocarbon consisting exclusively of a series of spiro-linked cyclopropane rings.

















