
A radiation dosimeter is a device that measures dose uptake of external ionizing radiation. It is worn by the person being monitored when used as a personal dosimeter, and is a record of the radiation dose received. Modern electronic personal dosimeters can give a continuous readout of cumulative dose and current dose rate, and can warn the wearer with an audible alarm when a specified dose rate or a cumulative dose is exceeded. Other dosimeters, such as thermoluminescent or film types, require processing after use to reveal the cumulative dose received, and cannot give a current indication of dose while being worn.

Luminescence is a spontaneous emission of radiation from an electronically or vibrationally excited species not in thermal equilibrium with its environment. A luminescent object emits cold light in contrast to incandescence, where an object only emits light after heating. Generally, the emission of light is due to the movement of electrons between different energy levels within an atom after excitation by external factors. However, the exact mechanism of light emission in vibrationally excited species is unknown.
Ionizing radiation, including nuclear radiation, consists of subatomic particles or electromagnetic waves that have sufficient energy to ionize atoms or molecules by detaching electrons from them. Some particles can travel up to 99% of the speed of light, and the electromagnetic waves are on the high-energy portion of the electromagnetic spectrum.

Thermoluminescence dating (TL) is the determination, by means of measuring the accumulated radiation dose, of the time elapsed since material containing crystalline minerals was either heated or exposed to sunlight (sediments). As a crystalline material is heated during measurements, the process of thermoluminescence starts. Thermoluminescence emits a weak light signal that is proportional to the radiation dose absorbed by the material. It is a type of luminescence dating.

Radiation dosimetry in the fields of health physics and radiation protection is the measurement, calculation and assessment of the ionizing radiation dose absorbed by an object, usually the human body. This applies both internally, due to ingested or inhaled radioactive substances, or externally due to irradiation by sources of radiation.
Radiation protection, also known as radiological protection, is defined by the International Atomic Energy Agency (IAEA) as "The protection of people from harmful effects of exposure to ionizing radiation, and the means for achieving this". Exposure can be from a source of radiation external to the human body or due to internal irradiation caused by the ingestion of radioactive contamination.
In physics, optically stimulated luminescence (OSL) is a method for measuring doses from ionizing radiation. It is used in at least two applications:

Thermoluminescence is a form of luminescence that is exhibited by certain crystalline materials, such as some minerals, when previously absorbed energy from electromagnetic radiation or other ionizing radiation is re-emitted as light upon heating of the material. The phenomenon is distinct from that of black-body radiation.

Health physics, also referred to as the science of radiation protection, is the profession devoted to protecting people and their environment from potential radiation hazards, while making it possible to enjoy the beneficial uses of radiation. Health physicists normally require a four-year bachelor’s degree and qualifying experience that demonstrates a professional knowledge of the theory and application of radiation protection principles and closely related sciences. Health physicists principally work at facilities where radionuclides or other sources of ionizing radiation are used or produced; these include research, industry, education, medical facilities, nuclear power, military, environmental protection, enforcement of government regulations, and decontamination and decommissioning—the combination of education and experience for health physicists depends on the specific field in which the health physicist is engaged.

An F-center or color center or Farbe center is a type of crystallographic defect in which an anionic vacancy in a crystal lattice is occupied by one or more unpaired electrons. Electrons in such a vacancy in a crystal lattice tend to absorb light in the visible spectrum such that a material that is usually transparent becomes colored. The greater the number of F centers, the more intense the color of the compound. F centers are a type of color center.
In radiation physics, kerma is an acronym for "kinetic energy released per unit mass", defined as the sum of the initial kinetic energies of all the charged particles liberated by uncharged ionizing radiation in a sample of matter, divided by the mass of the sample. It is defined by the quotient .
Irradiation is the process by which an object is exposed to radiation. An irradiator is a device used to expose an object to radiation, notably gamma radiation, for a variety of purposes. Irradiators may be used for sterilizing medical and pharmaceutical supplies, preserving foodstuffs, alteration of gemstone colors, studying radiation effects, eradicating insects through sterile male release programs, or calibrating thermoluminescent dosimeters (TLDs).
The ionization chamber is the simplest type of gaseous ionisation detector, and is widely used for the detection and measurement of many types of ionizing radiation, including X-rays, gamma rays, alpha particles and beta particles. Conventionally, the term "ionization chamber" refers exclusively to those detectors which collect all the charges created by direct ionization within the gas through the application of an electric field. It uses the discrete charges created by each interaction between the incident radiation and the gas to produce an output in the form of a small direct current. This means individual ionising events cannot be measured, so the energy of different types of radiation cannot be differentiated, but it gives a very good measurement of overall ionising effect.

A film badge dosimeter or film badge is a personal dosimeter used for monitoring cumulative radiation dose due to ionizing radiation.

Lithium fluoride is an inorganic compound with the chemical formula LiF. It is a colorless solid that transitions to white with decreasing crystal size. Its structure is analogous to that of sodium chloride, but it is much less soluble in water. It is mainly used as a component of molten salts. Partly because Li and F are both light elements, and partly because F2 is highly reactive, formation of LiF from the elements releases one of the highest energies per mass of reactants, second only to that of BeO.
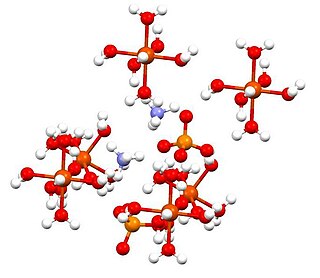
Ammonium iron(II) sulfate, or Mohr's salt, is the inorganic compound with the formula (NH4)2SO4.Fe(SO4).6H2O. Containing two different cations, Fe2+ and NH+4, it is classified as a double salt of ferrous sulfate and ammonium sulfate. It is a common laboratory reagent because it is readily crystallized, and crystals resist oxidation by air. Like the other ferrous sulfate salts, ferrous ammonium sulfate dissolves in water to give the aquo complex [Fe(H2O)6]2+, which has octahedral molecular geometry. Its mineral form is mohrite.

X-ray detectors are devices used to measure the flux, spatial distribution, spectrum, and/or other properties of X-rays.
Gel dosimeters, also called Fricke gel dosimeters, are manufactured from radiation sensitive chemicals that, upon irradiation with ionising radiation, undergo a fundamental change in their properties as a function of the absorbed radiation dose.

The electronic personal dosimeter (EPD) is a modern electronic dosimeter for estimating uptake of ionising radiation dose of the individual wearing it for radiation protection purposes. The electronic personal dosimeter has the advantages over older types that it has a number of sophisticated functions, such as continuous monitoring which allows alarm warnings at preset levels and live readout of dose accumulated. It can be reset to zero after use, and most models allow near field electronic communications for automatic reading and resetting.

Shakardokht (Shakar) Jafari is a Medical Physicist and an award-winning innovator based at the Surrey Technology Centre. She developed an efficient and low-cost method of measuring a medical dose of radiation.












