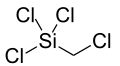
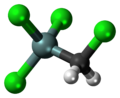
Chlorine is a chemical element with the symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between them. Chlorine is a yellow-green gas at room temperature. It is an extremely reactive element and a strong oxidising agent: among the elements, it has the highest electron affinity and the third-highest electronegativity on the Pauling scale, behind only oxygen and fluorine.
The chloride ion is the anion Cl−. It is formed when the element chlorine gains an electron or when a compound such as hydrogen chloride is dissolved in water or other polar solvents. Chloride salts such as sodium chloride are often very soluble in water. It is an essential electrolyte located in all body fluids responsible for maintaining acid/base balance, transmitting nerve impulses and regulating fluid in and out of cells. Less frequently, the word chloride may also form part of the "common" name of chemical compounds in which one or more chlorine atoms are covalently bonded. For example, methyl chloride, with the standard name chloromethane is an organic compound with a covalent C−Cl bond in which the chlorine is not an anion.

Brine is a high-concentration solution of salt (NaCl) in water (H2O). In different contexts, brine may refer to salt solutions ranging from about 3.5% (a typical concentration of seawater, on the lower end of solutions used for brining foods) up to about 26% (a typical saturated solution, depending on temperature). Lower levels of concentration are called by different names: fresh water, brackish water, and saline water.

Sodium chloride, commonly known as salt, is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. With molar masses of 22.99 and 35.45 g/mol respectively, 100 g of NaCl contains 39.34 g Na and 60.66 g Cl. Sodium chloride is the salt most responsible for the salinity of seawater and of the extracellular fluid of many multicellular organisms. In its edible form of table salt, it is commonly used as a condiment and food preservative. Large quantities of sodium chloride are used in many industrial processes, and it is a major source of sodium and chlorine compounds used as feedstocks for further chemical syntheses. A second major application of sodium chloride is de-icing of roadways in sub-freezing weather.
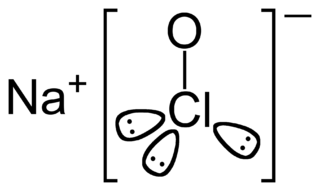
Sodium hypochlorite is a chemical compound with the formula NaOCl or NaClO, comprising a sodium cation and a hypochlorite anion. It may also be viewed as the sodium salt of hypochlorous acid. The anhydrous compound is unstable and may decompose explosively. It can be crystallized as a pentahydrate NaOCl·5H
2O, a pale greenish-yellow solid which is not explosive and is stable if kept refrigerated.
A halide is a binary phase, of which one part is a halogen atom and the other part is an element or radical that is less electronegative than the halogen, to make a, e.g., fluoride, chloride, or theoretically tennesside compound. The alkali metals combine directly with halogens under appropriate conditions forming halides of the general formula, MX. Many salts are halides; the hal- syllable in halide and halite reflects this correlation. All Group 1 metals form halides that are white solids at room temperature.

Iron(III) chloride is the inorganic compound with the formula. Also called ferric chloride, it is a common compound of iron in the +3 oxidation state. The anhydrous compound is a crystalline solid with a melting point of 307.6 °C. The color depends on the viewing angle: by reflected light the crystals appear dark green, but by transmitted light they appear purple-red.

Chlorine dioxide is a chemical compound with the formula ClO2 that exists as yellowish-green gas above 11 °C, a reddish-brown liquid between 11 °C and −59 °C, and as bright orange crystals below −59 °C. It is an oxidizing agent, able to transfer oxygen to a variety of substrates, while gaining one or more electrons via oxidation-reduction (redox). It does not hydrolyze when it enters water, and is usually handled as a dissolved gas in solution in water. Potential hazards with chlorine dioxide include health concerns, explosiveness and fire ignition. It is commonly used as a bleach.

The Bombardier Challenger 600 series is a family of business jets developed by Canadair after a Bill Lear concept, and then produced from 1986 by its new owner, Bombardier Aerospace. At the end of 1975, Canadair began funding the development of LearStar 600, and then bought the design for a wide-cabin business jet in April 1976. On 29 October, the programme was launched, backed by the Canadian federal government, and designed to comply with new FAR part 25 standards.

Titanium tetrachloride is the inorganic compound with the formula TiCl4. It is an important intermediate in the production of titanium metal and the pigment titanium dioxide. TiCl4 is a volatile liquid. Upon contact with humid air, it forms spectacular opaque clouds of titanium dioxide (TiO2) and hydrated hydrogen chloride. It is sometimes referred to as "tickle" or "tickle 4" due to the phonetic resemblance of its molecular formula (TiCl4) to the word.

Silver chloride is a chemical compound with the chemical formula AgCl. This white crystalline solid is well known for its low solubility in water (this behavior being reminiscent of the chlorides of Tl+ and Pb2+). Upon illumination or heating, silver chloride converts to silver (and chlorine), which is signaled by grey to black or purplish coloration to some samples. AgCl occurs naturally as a mineral chlorargyrite.

Dichlorine monoxide is an inorganic compound with the molecular formula Cl2O. It was first synthesised in 1834 by Antoine Jérôme Balard, who along with Gay-Lussac also determined its composition. In older literature it is often referred to as chlorine monoxide, which can be a source of confusion as that name now refers to the neutral species ClO.
Common Logic (CL) is a framework for a family of logic languages, based on first-order logic, intended to facilitate the exchange and transmission of knowledge in computer-based systems.
Chlorine (17Cl) has 25 isotopes with mass numbers ranging from 28Cl to 52Cl and 2 isomers. There are two stable isotopes, 35Cl (75.77%) and 37Cl (24.23%), giving chlorine a standard atomic weight of 35.45. The longest-lived radioactive isotope is 36Cl, which has a half-life of 301,000 years. All other isotopes have half-lives under 1 hour, many less than one second. The shortest-lived are 29Cl and 30Cl, with half-lives less than 10 picoseconds and 30 nanoseconds, respectively—the half-life of 28Cl is unknown.

OpenCL is a framework for writing programs that execute across heterogeneous platforms consisting of central processing units (CPUs), graphics processing units (GPUs), digital signal processors (DSPs), field-programmable gate arrays (FPGAs) and other processors or hardware accelerators. OpenCL specifies programming languages for programming these devices and application programming interfaces (APIs) to control the platform and execute programs on the compute devices. OpenCL provides a standard interface for parallel computing using task- and data-based parallelism.

Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid. It is a component of the gastric acid in the digestive systems of most animal species, including humans. Hydrochloric acid is an important laboratory reagent and industrial chemical.
Google Native Client (NaCl) is a sandboxing technology for running either a subset of Intel x86, ARM, or MIPS native code, or a portable executable, in a sandbox. It allows safely running native code from a web browser, independent of the user operating system, allowing web apps to run at near-native speeds, which aligns with Google's plans for Chrome OS. It may also be used for securing browser plugins, and parts of other applications or full applications such as ZeroVM.

Lee Chae-rin, better known by her stage name CL, is a South Korean rapper, singer and songwriter. Born in Seoul, South Korea, she spent much of her early life in Japan and France. CL trained at JYP Entertainment before joining YG Entertainment at the age of fifteen and debuted as a member of the girl group 2NE1 in 2009. The group went on to become one of South Korea's most popular and best-selling girl groups worldwide. She made her solo debut with the single "The Baddest Female" on May 28, 2013.