Ferrocene is an organometallic compound with the formula Fe(C5H5)2. The molecule is a complex consisting of two cyclopentadienyl rings sandwiching a central iron atom. It is an orange solid with a camphor-like odor that sublimes above room temperature, and is soluble in most organic solvents. It is remarkable for its stability: it is unaffected by air, water, strong bases, and can be heated to 400 °C without decomposition. In oxidizing conditions it can reversibly react with strong acids to form the ferrocenium cation Fe(C5H5)+2. Ferrocene and the ferrocenium cation are sometimes abbreviated as Fc and Fc+ respectively.

A lithium polymer battery, or more correctly lithium-ion polymer battery, is a rechargeable battery of lithium-ion technology using a polymer electrolyte instead of a liquid electrolyte. Highly conductive semisolid (gel) polymers form this electrolyte. These batteries provide higher specific energy than other lithium battery types and are used in applications where weight is a critical feature, such as mobile devices, radio-controlled aircraft and some electric vehicles.
In polymer chemistry, ring-opening polymerization (ROP) is a form of chain-growth polymerization in which the terminus of a polymer chain attacks cyclic monomers to form a longer polymer. The reactive center can be radical, anionic or cationic. Some cyclic monomers such as norbornene or cyclooctadiene can be polymerized to high molecular weight polymers by using metal catalysts. ROP is a versatile method for the synthesis of biopolymers.

Polyvinylidene fluoride or polyvinylidene difluoride (PVDF) is a highly non-reactive thermoplastic fluoropolymer produced by the polymerization of vinylidene difluoride. Its chemical formula is (C2H2F2)n.
Iron(III) chloride describes the inorganic compounds with the formula FeCl3(H2O)x. Also called ferric chloride, these compounds are some of the most important and commonplace compounds of iron. They are available both in anhydrous and in hydrated forms which are both hygroscopic. They feature iron in its +3 oxidation state. The anhydrous derivative is a Lewis acid, while all forms are mild oxidizing agents. It is used as a water cleaner and as an etchant for metals.

Polythiophenes (PTs) are polymerized thiophenes, a sulfur heterocycle. The parent PT is an insoluble colored solid with the formula (C4H2S)n. The rings are linked through the 2- and 5-positions. Poly(alkylthiophene)s have alkyl substituents at the 3- or 4-position(s). They are also colored solids, but tend to be soluble in organic solvents.

Polypyrrole (PPy) is an organic polymer obtained by oxidative polymerization of pyrrole. It is a solid with the formula H(C4H2NH)nH. It is an intrinsically conducting polymer, used in electronics, optical, biological and medical fields.
A polycatenane is a chemical substance that, like polymers, is chemically constituted by a large number of units. These units are made up of concatenated rings into a chain-like structure.
The Passerini reaction is a chemical reaction involving an isocyanide, an aldehyde, and a carboxylic acid to form a α-acyloxy amide. This addition reaction is one of the oldest isocyanide-based multicomponent reactions and was first described in 1921 by Mario Passerini in Florence, Italy. It is typically carried out in aprotic solvents but can also be performed in ionic liquids such as water or deep eutectic solvents. It is a third order reaction; first order in each of the reactants. The Passerini reaction is often used in combinatorial and medicinal chemistry with recent utility in green chemistry and polymer chemistry. As isocyanides exhibit high functional group tolerance, chemoselectivity, regioselectivity, and stereoselectivity, the Passerini reaction has a wide range of synthetic applications.
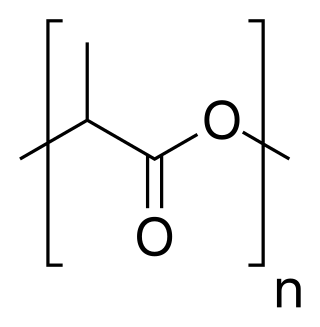
Polylactic acid, also known as poly(lactic acid) or polylactide (PLA), is a thermoplastic polyester with backbone formula (C
3H
4O
2)
n or [–C(CH
3)HC(=O)O–]
n, formally obtained by condensation of lactic acid C(CH
3)(OH)HCOOH with loss of water. It can also be prepared by ring-opening polymerization of lactide [–C(CH
3)HC(=O)O–]
2, the cyclic dimer of the basic repeating unit.

Iron(II) acetate is a coordination complex with formula Fe(CH3COO)2. It is a white solid, although impure samples can be slightly colored. A light green tetrahydrate is also known, which is highly soluble in water.

Nanocomposite is a multiphase solid material where one of the phases has one, two or three dimensions of less than 100 nanometers (nm) or structures having nano-scale repeat distances between the different phases that make up the material.

Temperature-responsive polymers or thermoresponsive polymers are polymers that exhibit drastic and discontinuous changes in their physical properties with temperature. The term is commonly used when the property concerned is solubility in a given solvent, but it may also be used when other properties are affected. Thermoresponsive polymers belong to the class of stimuli-responsive materials, in contrast to temperature-sensitive materials, which change their properties continuously with environmental conditions. In a stricter sense, thermoresponsive polymers display a miscibility gap in their temperature-composition diagram. Depending on whether the miscibility gap is found at high or low temperatures, either an upper critical solution temperature (UCST) or a lower critical solution temperature (LCST) exists.

The thiol-yne reaction is an organic reaction between a thiol and an alkyne. The reaction product is an alkenyl sulfide. The reaction was first reported in 1949 with thioacetic acid as reagent and rediscovered in 2009. It is used in click chemistry and in polymerization, especially with dendrimers.

Polyfluorene is a polymer with formula (C13H8)n, consisting of fluorene units linked in a linear chain — specifically, at carbon atoms 2 and 7 in the standard fluorene numbering. It can also be described as a chain of benzene rings linked in para positions with an extra methylene bridge connecting every pair of rings.

Iron(I) hydride, systematically named iron hydride and poly(hydridoiron) is a solid inorganic compound with the chemical formula (FeH)
n (also written ([FeH])
n or FeH). It is both thermodynamically and kinetically unstable toward decomposition at ambient temperature, and as such, little is known about its bulk properties.
Iron(II) hydride, systematically named iron dihydride and poly(dihydridoiron) is solid inorganic compound with the chemical formula (FeH
2)
n (also written ([FeH
2])n or FeH
2). ). It is kinetically unstable at ambient temperature, and as such, little is known about its bulk properties. However, it is known as a black, amorphous powder, which was synthesised for the first time in 2014.
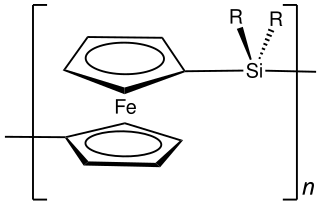
Polyferrocenes are polymers containing ferrocene units. Ferrocene offers many advantages over pure hydrocarbons when used as a building block of macromolecular chemistry. The variety of possible substitutions at the ferrocene parent body results in a multitude of accessible polymers with interesting electronic and photonic properties. Many polyferrocenes are relatively easily accessible. Poly(1,1'-ferrocene-silane) can be prepared by ring-opening polymerization and has a variety of interesting properties, such as a high refractive index or semiconductor properties. Ring-opening polymerization usually leads to polymers containing ferrocene in the backbone. Besides the latter motif, ferrocene can be attached to the backbone as pendant unit as well.

Pentaerythritol tetraacrylate is an organic compound. It is a tetrafunctional acrylate ester used as a monomer in the manufacture of polymers. As it is a polymerizable acrylate monomer, it is nearly always supplied with an added polymerisation inhibitor, such as MEHQ.
Vitaliy Khutoryanskiy FRSC is a British and Kazakhstani scientist, a Professor of Formulation Science and a Royal Society Industry Fellow at the University of Reading. His research focuses on polymers, biomaterials, nanomaterials, drug delivery, and pharmaceutical sciences. Khutoryanskiy has published over 200 original research articles, book chapters, and reviews. His publications have attracted > 11000 citations and his current h-index is 51. He received several prestigious awards in recognition for his research in polymers, colloids and drug delivery as well as for contributions to research peer-review and mentoring of early career researchers. He holds several honorary professorship titles from different universities.