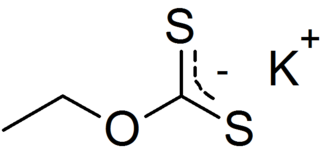In chemistry, an ester is a compound derived from an oxoacid in which at least one hydroxyl group is replaced by an alkoxy group, as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides are fatty acid esters of glycerol; they are important in biology, being one of the main classes of lipids and comprising the bulk of animal fats and vegetable oils.

Ethyl acetate is the organic compound with the formula CH3CO2CH2CH3, simplified to C4H8O2. This colorless liquid has a characteristic sweet smell and is used in glues, nail polish removers, and in the decaffeination process of tea and coffee. Ethyl acetate is the ester of ethanol and acetic acid; it is manufactured on a large scale for use as a solvent.
In organic chemistry, the Michael reaction or Michael addition is a reaction between a Michael donor and a Michael acceptor to produce a Michael adduct by creating a carbon-carbon bond at the acceptor's β-carbon. It belongs to the larger class of conjugate additions and is widely used for the mild formation of carbon-carbon bonds.
The Claisen condensation is a carbon–carbon bond forming reaction that occurs between two esters or one ester and another carbonyl compound in the presence of a strong base, resulting in a β-keto ester or a β-diketone. It is named after Rainer Ludwig Claisen, who first published his work on the reaction in 1887.

Xanthate usually refers to a salt with the formula ROCS−
2M+
(R = alkyl; M+ = Na+, K+), thus they are the metal-thioate/O-esters of dithiocarbonate. The name xanthates is derived from Ancient Greek ξανθός xanthos, meaning “yellowish, golden”, and indeed most xanthate salts are yellow. They were discovered and named in 1823 by Danish chemist William Christopher Zeise. These organosulfur compounds are important in two areas: the production of cellophane and related polymers from cellulose and (in mining) for extraction of certain ores. They are also versatile intermediates in organic synthesis. Xanthate can also refer to the O,S-ester of xanthic acid. These esters have the structure ROC(=S)SR′.

The Knorr pyrrole synthesis is a widely used chemical reaction that synthesizes substituted pyrroles (3). The method involves the reaction of an α-amino-ketone (1) and a compound containing an electron-withdrawing group α to a carbonyl group (2).
Sodium ethoxide, also referred to as sodium ethylate, is the ionic, organic compound with the formula C2H5ONa, or NaOEt. It is a white solid, although impure samples appear yellow or brown. It dissolves in polar solvents such as ethanol. It is commonly used as a strong base.
The Reformatsky reaction is an organic reaction which condenses aldehydes or ketones with α-halo esters using metallic zinc to form β-hydroxy-esters:
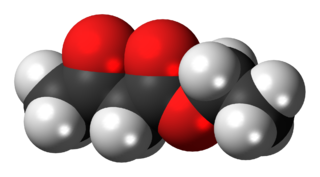
The organic compound ethyl acetoacetate (EAA) is the ethyl ester of acetoacetic acid. It is a colorless liquid. It is widely used as a chemical intermediate in the production of a wide variety of compounds. It is used as a flavoring for food.

Ethyl bromoacetate is the chemical compound with the formula CH2BrCO2C2H5. It is the ethyl ester of bromoacetic acid and is prepared in two steps from acetic acid. It is a lachrymator and has a fruity, pungent odor. It is also a highly toxic alkylating agent and may be fatal if inhaled.
Cycloheptatriene (CHT) is an organic compound with the formula C7H8. It is a closed ring of seven carbon atoms joined by three double bonds (as the name implies) and four single bonds. This colourless liquid has been of recurring theoretical interest in organic chemistry. It is a ligand in organometallic chemistry and a building block in organic synthesis. Cycloheptatriene is not aromatic, as reflected by the nonplanarity of the methylene bridge (-CH2-) with respect to the other atoms; however the related tropylium cation is.

Trimethylsilyl chloride, also known as chlorotrimethylsilane is an organosilicon compound (silyl halide), with the formula (CH3)3SiCl, often abbreviated Me3SiCl or TMSCl. It is a colourless volatile liquid that is stable in the absence of water. It is widely used in organic chemistry.
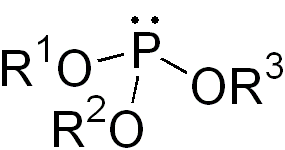
In organic chemistry, a phosphite ester or organophosphite usually refers to an organophosphorous compound with the formula P(OR)3. They can be considered as esters of an unobserved tautomer phosphorous acid, H3PO3, with the simplest example being trimethylphosphite, P(OCH3)3. Some phosphites can be considered esters of the dominant tautomer of phosphorous acid (HP(O)(OH)2). The simplest representative is dimethylphosphite with the formula HP(O)(OCH3)2. Both classes of phosphites are usually colorless liquids.

In organic chemistry, alkyl nitrites are a group of organic compounds based upon the molecular structure R−O−N=O, where R represents an alkyl group. Formally they are alkyl esters of nitrous acid. They are distinct from nitro compounds.
Acetoacetic ester synthesis is a chemical reaction where ethyl acetoacetate is alkylated at the α-carbon to both carbonyl groups and then converted into a ketone, or more specifically an α-substituted acetone. This is very similar to malonic ester synthesis.
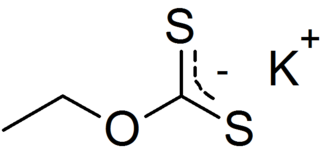
Potassium ethyl xanthate (KEX) is an organosulfur compound with the chemical formula CH3CH2OCS2K. It is a pale yellow powder that is used in the mining industry for the separation of ores. Unlike the related sodium ethyl xanthate, the potassium salt exists as an anhydrous salt.
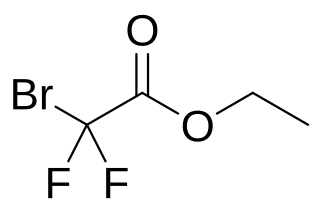
Ethyl bromodifluoroacetate is an ester that can be used to introduce the CF2 group when synthesising chemical compounds. It is a clear to yellow liquid. It is an ester of bromodifluoroacetic acid and ethyl alcohol.

Ethyl pyruvate is a colorless organic compound with a molecular formula of C5H8O3.
In chemistry, a thioxanthate is an organosulfur compound with the formula RSCS2X. When X is an alkali metal, the thioxanthate is a salt. When X is a transition metal, the thioxanthate is a ligand, and when X is an organic group, the compounds are called thioxanthate esters. They are usually yellow colored compounds that often dissolve in organic solvents. They are used as precursors to some catalysts, froth flotation agents, and additives for lubricants.

Ethyl propiolate is an organic compound with the formula HC2CO2C2H5. It is the ethyl ester of propiolic acid, the simplest acetylenic carboxylic acid. It is a colorless liquid that is miscible with organic solvents. The compound is a reagent and building block for the synthesis of other organic compounds, reactions that exploit the electrophilicity of the alkyne group.