Related Research Articles

Cytosine is one of the four nucleobases found in DNA and RNA, along with adenine, guanine, and thymine. It is a pyrimidine derivative, with a heterocyclic aromatic ring and two substituents attached. The nucleoside of cytosine is cytidine. In Watson-Crick base pairing, it forms three hydrogen bonds with guanine.

Deoxyribonucleic acid is a polymer composed of two polynucleotide chains that coil around each other to form a double helix carrying genetic instructions for the development, functioning, growth and reproduction of all known organisms and many viruses. DNA and ribonucleic acid (RNA) are nucleic acids. Alongside proteins, lipids and complex carbohydrates (polysaccharides), nucleic acids are one of the four major types of macromolecules that are essential for all known forms of life.
In the chemical sciences, methylation denotes the addition of a methyl group on a substrate, or the substitution of an atom by a methyl group. Methylation is a form of alkylation, with a methyl group replacing a hydrogen atom. These terms are commonly used in chemistry, biochemistry, soil science, and the biological sciences.
Deamination is the removal of an amino group from a molecule. Enzymes that catalyse this reaction are called deaminases.

5-Methylcytosine is a methylated form of the DNA base cytosine (C) that regulates gene transcription and takes several other biological roles. When cytosine is methylated, the DNA maintains the same sequence, but the expression of methylated genes can be altered. 5-Methylcytosine is incorporated in the nucleoside 5-methylcytidine.

The CpG sites or CG sites are regions of DNA where a cytosine nucleotide is followed by a guanine nucleotide in the linear sequence of bases along its 5' → 3' direction. CpG sites occur with high frequency in genomic regions called CpG islands.
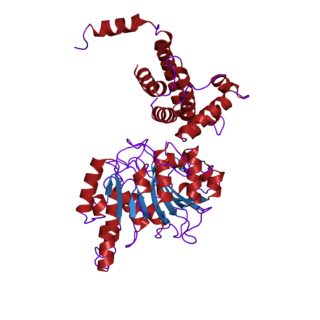
In biochemistry, the DNA methyltransferase family of enzymes catalyze the transfer of a methyl group to DNA. DNA methylation serves a wide variety of biological functions. All the known DNA methyltransferases use S-adenosyl methionine (SAM) as the methyl donor.

In biochemistry, a ribonucleotide is a nucleotide containing ribose as its pentose component. It is considered a molecular precursor of nucleic acids. Nucleotides are the basic building blocks of DNA and RNA. Ribonucleotides themselves are basic monomeric building blocks for RNA. Deoxyribonucleotides, formed by reducing ribonucleotides with the enzyme ribonucleotide reductase (RNR), are essential building blocks for DNA. There are several differences between DNA deoxyribonucleotides and RNA ribonucleotides. Successive nucleotides are linked together via phosphodiester bonds.

DNA methylation is a biological process by which methyl groups are added to the DNA molecule. Methylation can change the activity of a DNA segment without changing the sequence. When located in a gene promoter, DNA methylation typically acts to repress gene transcription. In mammals, DNA methylation is essential for normal development and is associated with a number of key processes including genomic imprinting, X-chromosome inactivation, repression of transposable elements, aging, and carcinogenesis.
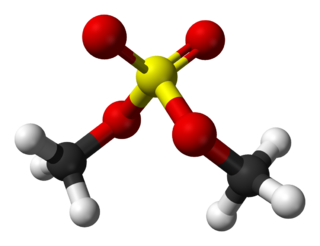
Dimethyl sulfate (DMS) is a chemical compound with formula (CH3O)2SO2. As the diester of methanol and sulfuric acid, its formula is often written as (CH3)2SO4 or Me2SO4, where CH3 or Me is methyl. Me2SO4 is mainly used as a methylating agent in organic synthesis.

The bisulfite ion (IUPAC-recommended nomenclature: hydrogensulfite) is the ion HSO−
3. Salts containing the HSO−
3 ion are also known as "sulfite lyes". Sodium bisulfite is used interchangeably with sodium metabisulfite (Na2S2O5). Sodium metabisulfite dissolves in water to give a solution of Na+HSO−
3.
In biology, reprogramming refers to erasure and remodeling of epigenetic marks, such as DNA methylation, during mammalian development or in cell culture. Such control is also often associated with alternative covalent modifications of histones.

Methylation specific oligonucleotide microarray, also known as MSO microarray, was developed as a technique to map epigenetic methylation changes in DNA of cancer cells.

Methyltransferases are a large group of enzymes that all methylate their substrates but can be split into several subclasses based on their structural features. The most common class of methyltransferases is class I, all of which contain a Rossmann fold for binding S-Adenosyl methionine (SAM). Class II methyltransferases contain a SET domain, which are exemplified by SET domain histone methyltransferases, and class III methyltransferases, which are membrane associated. Methyltransferases can also be grouped as different types utilizing different substrates in methyl transfer reactions. These types include protein methyltransferases, DNA/RNA methyltransferases, natural product methyltransferases, and non-SAM dependent methyltransferases. SAM is the classical methyl donor for methyltransferases, however, examples of other methyl donors are seen in nature. The general mechanism for methyl transfer is a SN2-like nucleophilic attack where the methionine sulfur serves as the leaving group and the methyl group attached to it acts as the electrophile that transfers the methyl group to the enzyme substrate. SAM is converted to S-Adenosyl homocysteine (SAH) during this process. The breaking of the SAM-methyl bond and the formation of the substrate-methyl bond happen nearly simultaneously. These enzymatic reactions are found in many pathways and are implicated in genetic diseases, cancer, and metabolic diseases. Another type of methyl transfer is the radical S-Adenosyl methionine (SAM) which is the methylation of unactivated carbon atoms in primary metabolites, proteins, lipids, and RNA.

Treat Baldwin Johnson was an American organic chemist and Sterling Professor at Yale University from 1928–1943.

Bisulfitesequencing (also known as bisulphite sequencing) is the use of bisulfite treatment of DNA before routine sequencing to determine the pattern of methylation. DNA methylation was the first discovered epigenetic mark, and remains the most studied. In animals it predominantly involves the addition of a methyl group to the carbon-5 position of cytosine residues of the dinucleotide CpG, and is implicated in repression of transcriptional activity.

For molecular biology in mammals, DNA demethylation causes replacement of 5-methylcytosine (5mC) in a DNA sequence by cytosine (C). DNA demethylation can occur by an active process at the site of a 5mC in a DNA sequence or, in replicating cells, by preventing addition of methyl groups to DNA so that the replicated DNA will largely have cytosine in the DNA sequence.

5-Hydroxymethylcytosine (5hmC) is a DNA pyrimidine nitrogen base derived from cytosine. It is potentially important in epigenetics, because the hydroxymethyl group on the cytosine can possibly switch a gene on and off. It was first seen in bacteriophages in 1952. However, in 2009 it was found to be abundant in human and mouse brains, as well as in embryonic stem cells. In mammals, it can be generated by oxidation of 5-methylcytosine, a reaction mediated by TET enzymes. Its molecular formula is C5H7N3O2.

DNA base flipping, or nucleotide flipping, is a mechanism in which a single nucleotide base, or nucleobase, is rotated outside the nucleic acid double helix. This occurs when a nucleic acid-processing enzyme needs access to the base to perform work on it, such as its excision for replacement with another base during DNA repair. It was first observed in 1994 using X-ray crystallography in a methyltransferase enzyme catalyzing methylation of a cytosine base in DNA. Since then, it has been shown to be used by different enzymes in many biological processes such as DNA methylation, various DNA repair mechanisms, and DNA replication. It can also occur in RNA double helices or in the DNA:RNA intermediates formed during RNA transcription.

Whole genome bisulfite sequencing is a next-generation sequencing technology used to determine the DNA methylation status of single cytosines by treating the DNA with sodium bisulfite before high-throughput DNA sequencing. The DNA methylation status at various genes can reveal information regarding gene regulation and transcriptional activities. This technique was developed in 2009 along with reduced representation bisulfite sequencing after bisulfite sequencing became the gold standard for DNA methylation analysis.
References
- ↑ Matthews AP (2012). Physiological Chemistry. Williams & Wilkins Company. p. 167. ISBN 978-1130145373.
- ↑ Linton DS (2005). Emil Von Behring: Infectious Disease, Immunology, Serum Therapy, Volume 255. American Philosophical Society, Philadelphia. pp. 265–266. ISBN 0871692554.
- ↑ Johnson, Treat B.; Coghill, Robert D. (1925). "The discovery of 5-methyl-cytosine in tuberculinic acid, the nucleic acid of the Tubercle bacillus". Journal of the American Chemical Society. 47 (11): 2838. doi:10.1021/ja01688a030.
- ↑ Grosjean H (2009). Nucleic Acids Are Not Boring Long Polymers of Only Four Types of Nucleotides: A Guided Tour. Landes Bioscience.
- 1 2 Vischer, E; Zamenhof, S; Chargaff, E (1949). "Microbial nucleic acids; the desoxypentose nucleic acids of avian tubercle bacilli and yeast". The Journal of Biological Chemistry. 177 (1): 429–38. doi: 10.1016/S0021-9258(18)57100-3 . PMID 18107446.
- ↑ Wyatt GR (1950). "Occurrence of 5-methylcytosine in nucleic acids". Nature. 166 (4214): 237–238. doi:10.1038/166237b0. PMID 15439258. S2CID 4215082.
- ↑ Neumann HP (2008). Progress in DNA Methylation Research. Nova. p. 190. ISBN 978-1600217227.