
A nickel–metal hydride battery is a type of rechargeable battery. The chemical reaction at the positive electrode is similar to that of the nickel–cadmium cell (NiCd), with both using nickel oxide hydroxide (NiOOH). However, the negative electrodes use a hydrogen-absorbing alloy instead of cadmium. NiMH batteries can have two to three times the capacity of NiCd batteries of the same size, with significantly higher energy density, although only about half that of lithium-ion batteries.

A lithium-ion or Li-ion battery is a type of rechargeable battery that uses the reversible intercalation of Li+ ions into electronically conducting solids to store energy. In comparison with other commercial rechargeable batteries, Li-ion batteries are characterized by higher specific energy, higher energy density, higher energy efficiency, a longer cycle life, and a longer calendar life. Also noteworthy is a dramatic improvement in lithium-ion battery properties after their market introduction in 1991: over the following 30 years, their volumetric energy density increased threefold while their cost dropped tenfold.

A rechargeable battery, storage battery, or secondary cell, is a type of electrical battery which can be charged, discharged into a load, and recharged many times, as opposed to a disposable or primary battery, which is supplied fully charged and discarded after use. It is composed of one or more electrochemical cells. The term "accumulator" is used as it accumulates and stores energy through a reversible electrochemical reaction. Rechargeable batteries are produced in many different shapes and sizes, ranging from button cells to megawatt systems connected to stabilize an electrical distribution network. Several different combinations of electrode materials and electrolytes are used, including lead–acid, zinc–air, nickel–cadmium (NiCd), nickel–metal hydride (NiMH), lithium-ion (Li-ion), lithium iron phosphate (LiFePO4), and lithium-ion polymer.

A lithium polymer battery, or more correctly, lithium-ion polymer battery, is a rechargeable battery of lithium-ion technology using a polymer electrolyte instead of a liquid electrolyte. Highly conductive semisolid (gel) polymers form this electrolyte. These batteries provide higher specific energy than other lithium battery types. They are used in applications where weight is critical, such as mobile devices, radio-controlled aircraft, and some electric vehicles.

An alkaline battery is a type of primary battery where the electrolyte has a pH value above 7. Typically these batteries derive energy from the reaction between zinc metal and manganese dioxide.

A dry cell is a type of electric battery, commonly used for portable electrical devices. Unlike wet cell batteries, which have a liquid electrolyte, dry cells use an electrolyte in the form of a paste, and are thus less susceptible to leakage.

A zinc–air battery is a metal–air electrochemical cell powered by the oxidation of zinc with oxygen from the air. During discharge, a mass of zinc particles forms a porous anode, which is saturated with an electrolyte. Oxygen from the air reacts at the cathode and forms hydroxyl ions which migrate into the zinc paste and form zincate, releasing electrons to travel to the cathode. The zincate decays into zinc oxide and water returns to the electrolyte. The water and hydroxyl from the anode are recycled at the cathode, so the water is not consumed. The reactions produce a theoretical voltage of 1.65 Volts, but is reduced to 1.35–1.4 V in available cells.

A zinc–carbon battery (or carbon zinc battery in U.S. English) is a dry cell primary battery that provides direct electric current from the electrochemical reaction between zinc (Zn) and manganese dioxide (MnO2) in the presence of an ammonium chloride (NH4Cl) electrolyte. It produces a voltage of about 1.5 volts between the zinc anode, which is typically constructed as a cylindrical container for the battery cell, and a carbon rod surrounded by a compound with a higher Standard electrode potential (positive polarity), known as the cathode, that collects the current from the manganese dioxide electrode. The name "zinc-carbon" is slightly misleading as it implies that carbon is acting as the oxidizing agent rather than the manganese dioxide.

Lithium metal batteries are primary batteries that have metallic lithium as an anode. The name intentionally refers to the metal as to distinguish them from lithium-ion batteries, which use lithiated metal oxides as the cathode material. Although most lithium metal batteries are non-rechargeable, rechargeable lithium metal batteries are also under development. Since 2007, Dangerous Goods Regulations differentiate between lithium metal batteries and lithium-ion batteries.

The nine-volt battery, or 9-volt battery, is an electric battery that supplies a nominal voltage of 9 volts. Actual voltage measures 7.2 to 9.6 volts, depending on battery chemistry. Batteries of various sizes and capacities are manufactured; a very common size is known as PP3, introduced for early transistor radios. The PP3 has a rectangular prism shape with rounded edges and two polarized snap connectors on the top. This type is commonly used for many applications including household uses such as smoke detectors, gas detectors, clocks, and toys.

The lithium iron phosphate battery or LFP battery is a type of lithium-ion battery using lithium iron phosphate as the cathode material, and a graphitic carbon electrode with a metallic backing as the anode. Because of their low cost, high safety, low toxicity, long cycle life and other factors, LFP batteries are finding a number of roles in vehicle use, utility-scale stationary applications, and backup power. LFP batteries are cobalt-free. As of September 2022, LFP type battery market share for EVs reached 31%, and of that, 68% were from EV makers Tesla and BYD alone. Chinese manufacturers currently hold a near monopoly of LFP battery type production. With patents having started to expire in 2022 and the increased demand for cheaper EV batteries, LFP type production is expected to rise further and surpass lithium nickel manganese cobalt oxides (NMC) type batteries in 2028.

Nanobatteries are fabricated batteries employing technology at the nanoscale, particles that measure less than 100 nanometers or 10−7 meters. These batteries may be nano in size or may use nanotechnology in a macro scale battery. Nanoscale batteries can be combined to function as a macrobattery such as within a nanopore battery.

Batteries provided the main source of electricity before the development of electric generators and electrical grids around the end of the 19th century. Successive improvements in battery technology facilitated major electrical advances, from early scientific studies to the rise of telegraphs and telephones, eventually leading to portable computers, mobile phones, electric cars, and many other electrical devices.

An electric battery is a source of electric power consisting of one or more electrochemical cells with external connections for powering electrical devices. When a battery is supplying power, its positive terminal is the cathode and its negative terminal is the anode. The terminal marked negative is the source of electrons. When a battery is connected to an external electric load, those negatively charged electrons flow through the circuit and reach to the positive terminal, thus cause a redox reaction by attracting positively charged ions, cations. Thus converts high-energy reactants to lower-energy products, and the free-energy difference is delivered to the external circuit as electrical energy. Historically the term "battery" specifically referred to a device composed of multiple cells; however, the usage has evolved to include devices composed of a single cell.
The thin-film lithium-ion battery is a form of solid-state battery. Its development is motivated by the prospect of combining the advantages of solid-state batteries with the advantages of thin-film manufacturing processes.
The lithium–air battery (Li–air) is a metal–air electrochemical cell or battery chemistry that uses oxidation of lithium at the anode and reduction of oxygen at the cathode to induce a current flow.
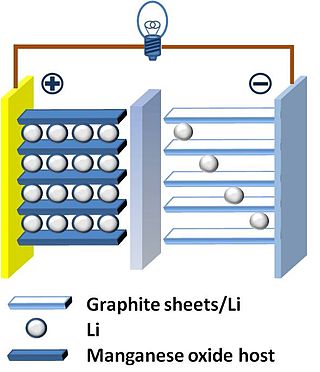
A lithium ion manganese oxide battery (LMO) is a lithium-ion cell that uses manganese dioxide, MnO
2, as the cathode material. They function through the same intercalation/de-intercalation mechanism as other commercialized secondary battery technologies, such as LiCoO
2. Cathodes based on manganese-oxide components are earth-abundant, inexpensive, non-toxic, and provide better thermal stability.
Research in lithium-ion batteries has produced many proposed refinements of lithium-ion batteries. Areas of research interest have focused on improving energy density, safety, rate capability, cycle durability, flexibility, and reducing cost.

This is a history of the lithium-ion battery.















