
Ultrasound is sound with frequencies greater than 20 kilohertz. This frequency is the approximate upper audible limit of human hearing in healthy young adults. The physical principles of acoustic waves apply to any frequency range, including ultrasound. Ultrasonic devices operate with frequencies from 20 kHz up to several gigahertz.

Echolocation, also called bio sonar, is a biological active sonar used by several animal groups, both in the air and underwater. Echolocating animals emit calls and listen to the echoes of those calls that return from various objects near them. They use these echoes to locate and identify the objects. Echolocation is used for navigation, foraging, and hunting prey.

Microbats constitute the suborder Microchiroptera within the order Chiroptera (bats). Bats have long been differentiated into Megachiroptera (megabats) and Microchiroptera, based on their size, the use of echolocation by the Microchiroptera and other features; molecular evidence suggests a somewhat different subdivision, as the microbats have been shown to be a paraphyletic group.

The Arctiinae are a large and diverse subfamily of moths with around 11,000 species found all over the world, including 6,000 neotropical species. This subfamily includes the groups commonly known as tiger moths, which usually have bright colours, footmen, which are usually much drabber, lichen moths, and wasp moths. Many species have "hairy" caterpillars that are popularly known as woolly bears or woolly worms. The scientific name Arctiinae refers to this hairiness. Some species within the Arctiinae have the word "tussock"' in their common names because they have been misidentified as members of the Lymantriinae subfamily based on the characteristics of the larvae.

Range fractionation is a term used in biology to describe the way by which a group of sensory neurons are able to encode varying magnitudes of a stimulus. Sense organs are usually composed of many sensory receptors measuring the same property. These sensory receptors show a limited degree of precision due to an upper limit in firing rate. If the receptors are endowed with distinct transfer functions in such a way that the points of highest sensitivity are scattered along the axis of the quality being measured, the precision of the sense organ as a whole can be increased.

Anti-predator adaptations are mechanisms developed through evolution that assist prey organisms in their constant struggle against predators. Throughout the animal kingdom, adaptations have evolved for every stage of this struggle, namely by avoiding detection, warding off attack, fighting back, or escaping when caught.
In evolutionary biology, an evolutionary arms race is an ongoing struggle between competing sets of co-evolving genes, phenotypic and behavioral traits that develop escalating adaptations and counter-adaptations against each other, resembling the geopolitical concept of an arms race. These are often described as examples of positive feedback. The co-evolving gene sets may be in different species, as in an evolutionary arms race between a predator species and its prey, or a parasite and its host. Alternatively, the arms race may be between members of the same species, as in the manipulation/sales resistance model of communication or as in runaway evolution or Red Queen effects. One example of an evolutionary arms race is in sexual conflict between the sexes, often described with the term Fisherian runaway. Thierry Lodé emphasized the role of such antagonistic interactions in evolution leading to character displacements and antagonistic coevolution.

Ormia ochracea is a small yellow nocturnal fly in the family Tachinidae. It is notable for its parasitism of crickets and its exceptionally acute directional hearing. The female is attracted to the song of the male cricket and deposits larvae on or around him, as was discovered in 1975 by the zoologist William H. Cade.

In ecology, crypsis is the ability of an animal or a plant to avoid observation or detection by other animals. It may be a predation strategy or an antipredator adaptation. Methods include camouflage, nocturnality, subterranean lifestyle and mimicry. Crypsis can involve visual, olfactory or auditory concealment. When it is visual, the term cryptic coloration, effectively a synonym for animal camouflage, is sometimes used, but many different methods of camouflage are employed by animals or plants.

Campaniform sensilla are a class of mechanoreceptors found in insects, which respond to local stress and strain within the animal's cuticle. Campaniform sensilla function as proprioceptors that detect mechanical load as resistance to muscle contraction, similar to mammalian Golgi tendon organs. Sensory feedback from campaniform sensilla is integrated in the control of posture and locomotion.
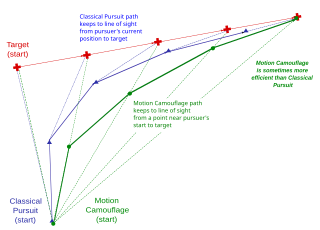
Motion camouflage is camouflage which provides a degree of concealment for a moving object, given that motion makes objects easy to detect however well their coloration matches their background or breaks up their outlines.

A tympanal organ is a hearing organ in insects, consisting of a membrane (tympanum) stretched across a frame backed by an air sac and associated sensory neurons. Sounds vibrate the membrane, and the vibrations are sensed by a chordotonal organ. Hymenoptera do not have a tympanal organ, but they do have a Johnston's organ.

Cycnia tenera, the dogbane tiger moth or delicate cycnia, is a moth in the family Erebidae. It occurs throughout North America, from southern British Columbia to Nova Scotia southwards to Arizona and Florida. The species is distasteful and there is evidence that it emits aposematic ultrasound signals; these may also jam bat echolocation, as the functions are not mutually exclusive.

Teleogryllus oceanicus, commonly known as the Australian, Pacific or oceanic field cricket, is a cricket found across Oceania and in coastal Australia from Carnarvon in Western Australia and Rockhampton in north-east Queensland
James A. Simmons is a pioneer in the field of biosonar. His research includes behavioral and neurophysiological studies of sound processing in the echolocating bat. From the time he began graduate research in the late 1960s to the present, he has been in the forefront of bat echolocation research. Simmons was honored as a fellow of the Acoustical Society of America (ASA) in 1996 and of the American Association for the Advancement of Science in 2000. He was awarded the ASA's second Silver Medal in Animal Bioacoustics in 2005. His current position is Professor in the Department of Neuroscience, Brown University.

Bats are flying mammals of the order Chiroptera. With their forelimbs adapted as wings, they are the only mammals capable of true and sustained flight. Bats are more agile in flight than most birds, flying with their very long spread-out digits covered with a thin membrane or patagium. The smallest bat, and arguably the smallest extant mammal, is Kitti's hog-nosed bat, which is 29–34 millimetres in length, 150 mm (6 in) across the wings and 2–2.6 g in mass. The largest bats are the flying foxes, with the giant golden-crowned flying fox reaching a weight of 1.6 kg and having a wingspan of 1.7 m.

Mantises are an order (Mantodea) of insects that contains over 2,400 species in about 460 genera in 33 families. The largest family is the Mantidae ("mantids"). Mantises are distributed worldwide in temperate and tropical habitats. They have triangular heads with bulging eyes supported on flexible necks. Their elongated bodies may or may not have wings, but all Mantodea have forelegs that are greatly enlarged and adapted for catching and gripping prey; their upright posture, while remaining stationary with forearms folded, has led to the common name praying mantis.

Deimatic behaviour or startle display means any pattern of bluffing behaviour in an animal that lacks strong defences, such as suddenly displaying conspicuous eyespots, to scare off or momentarily distract a predator, thus giving the prey animal an opportunity to escape. The term deimatic or dymantic originates from the Greek δειματόω (deimatóo), meaning "to frighten".
Echolocation systems of animals, like human radar systems, are susceptible to interference known as echolocation jamming or sonar jamming. Jamming occurs when non-target sounds interfere with target echoes. Jamming can be purposeful or inadvertent, and can be caused by the echolocation system itself, other echolocating animals, prey, or humans. Echolocating animals have evolved to minimize jamming, however; echolocation avoidance behaviors are not always successful.
Rohini Balakrishnan is an Indian bioacoustics expert. She is a senior Professor and Chair of the Centre for Ecological Sciences at the Indian Institute of Science (IISc), Bengaluru. Her research focuses on animal behavior through the lens of animal communication and bioacoustics.
















