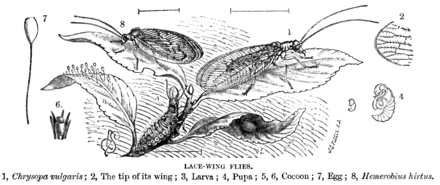
Green lacewings are insects in the large family Chrysopidae of the order Neuroptera. There are about 85 genera and 1,300–2,000 species in this widespread group. Members of the genera Chrysopa and Chrysoperla are very common in North America and Europe; they are very similar and many of their species have been moved from one genus to the other time and again, and in the nonscientific literature assignment to Chrysopa and Chrysoperla can rarely be relied upon. Since they are the most familiar neuropterans to many people, they are often simply called "lacewings". Since most of the diversity of Neuroptera are properly referred to as some sort of "lacewing", common lacewings is preferable.

Megaloptera is an order of insects. It contains the alderflies, dobsonflies and fishflies, and there are about 300 known species.

Snakeflies are a group of predatory insects comprising the order Raphidioptera with two extant families: Raphidiidae and Inocelliidae, consisting of roughly 260 species. In the past, the group had a much wider distribution than it does now; snakeflies are found in temperate regions worldwide but are absent from the tropics and the Southern Hemisphere. Recognisable representatives of the group first appeared during the Early Jurassic. They are a relict group, having reached their apex of diversity during the Cretaceous before undergoing substantial decline.

Mantispidae, known commonly as mantidflies, mantispids, mantid lacewings, mantisflies or mantis-flies, is a family of small to moderate-sized insects in the order Neuroptera. There are many genera with around 400 species worldwide, especially in the tropics and subtropics. Only five species of Mantispa occur in Europe. As their names suggest, members of the group possess raptorial forelimbs similar to those of the praying mantis, a case of convergent evolution.

Ascalaphidae is a family of insects in the order Neuroptera, commonly called owlflies; there are some 450 extant species. They are fast-flying crepuscular or diurnal predators of other flying insects, and have large bulging eyes and strongly knobbed antennae. The larvae are ambush predators; some of them make use of self-decoration camouflage.

Osmylidae are a small family of winged insects of the net-winged insect order Neuroptera. The osmylids, also called lance lacewings, stream lacewings or giant lacewings, are found all over the world except North and Central America. There are around 225 extant species.

Hemerobiidae is a family of Neuropteran insects commonly known as brown lacewings, comprising about 500 species in 28 genera. Most are yellow to dark brown, but some species are green. They are small; most have forewings 4–10 mm long. These insects differ from the somewhat similar Chrysopidae not only by the usual coloring but also by the wing venation: hemerobiids differ from chrysopids in having numerous long veins and forked costal cross veins. Some genera are widespread, but most are restricted to a single biogeographical realm. Some species have reduced wings to the degree that they are flightless. Imagines (adults) of subfamily Drepanepteryginae mimic dead leaves. Hemerobiid larvae are usually less hairy than chrysopid larvae.

The Neuropterida are a clade, sometimes placed at superorder level, of holometabolous insects with over 5,700 described species, containing the orders Neuroptera, Megaloptera, and Raphidioptera (snakeflies).

The Berothidae are a family of winged insects of the order Neuroptera. They are known commonly as the beaded lacewings. The family was first named by Anton Handlirsch in 1906. The family consists of 24 genera and 110 living species distributed discontinuously worldwide, mostly in tropical and subtropical regions. Numerous extinct species have also been described. Their ecology is poorly known, but in the species where larval stages have been documented, the larvae are predators of termites.

The Nevrorthidae are a small family of lacewings in the order Neuroptera. There are 19 extant species in four genera, with a geographically disjunct distribution: Nevrorthus, comprising 5 species with scattered distributions around the Mediterranean; Austroneurorthus, with two species known from southeastern Australia; Nipponeurorthus, comprising 11 species known from China and Japan; and Sinoneurorthus, known from a single species described from Yunnan Province, China. They are traditionally placed in the Osmyloidea, alongside Osmylidae and the spongillaflies (Sisyridae), but some research has considered them to be the sister group to the rest of Neuroptera. The larvae have unique straight jaws that are curved at the tips, and live as unspecialised predators in the sandy bottom sediments of clear, fast flowing mountain rivers and streams. They pupate underwater on the underside of stones. The adults are likely predators or feed on honeydew and other sugar-rich fluids.

Nymphidae, sometimes called split-footed lacewings, are a family of winged insects of the order Neuroptera. There are 35 extant species native to Australia and New Guinea.

Psychopsidae is a family of winged insects of the order Neuroptera. They are commonly called silky lacewings.

Sisyridae, commonly known as spongeflies or spongillaflies, are a family of winged insects in the order Neuroptera. There are approximately 60 living species described, and several extinct species identified from the fossil record.

The dustywings, Coniopterygidae, are a family of Pterygota of the net-winged insect order (Neuroptera). About 460 living species are known. These tiny insects can usually be determined to genus with a hand lens according to their wing venation, but to distinguish species, examination of the genitals by microscope is usually necessary.

Chrysopa is a genus of green lacewings in the neuropteran family Chrysopidae.

Osmyloidea is a euneuropteran superfamily in the lacewing order Neuroptera sister to the superfamilies Dilaroidea, Mantispoidea, and the clade Neoneuroptera. The superfamily includes three living families and two extinct families described from the fossil record.

Myrmeleontoidea is a neuropteran superfamily in the clade Myrmeleontiformia. Engel, Winteron, and Breitkreuz (2018) included the following families:

Myrmeleontiformia is an insect clade in the order Neuroptera, and which was historically treated as a suborder. The phylogeny of the Neuroptera has been explored using mitochondrial DNA sequences, and while issues remain for the order as a whole, such as "Hemerobiiformia" being paraphyletic, Myrmeleontiformia is generally agreed to be monophyletic, with one study giving the following cladogram:

Dipteromantispidae is an extinct family of neuropterans known from the Cretaceous period. Unlike other neuropterans, the family possesses only a single set of fully developed forewings, with the hindwings reduced to haltere-like structures. They are generally small in size and possess raptorial forelegs. They are considered to belong to Mantispoidea, with an uncertain position within the clade. Some authors have suggested that they represent a subgroup of Mantispidae, and should instead be referred to as the subfamily Dipteromantispinae within that family.