Related Research Articles
Prader–Willi syndrome (PWS) is a genetic disorder caused by a loss of function of specific genes on chromosome 15. In newborns, symptoms include weak muscles, poor feeding, and slow development. Beginning in childhood, those affected become constantly hungry, which often leads to obesity and type 2 diabetes. Mild to moderate intellectual impairment and behavioral problems are also typical of the disorder. Often, affected individuals have a narrow forehead, small hands and feet, short height, and light skin and hair. Most are unable to have children.
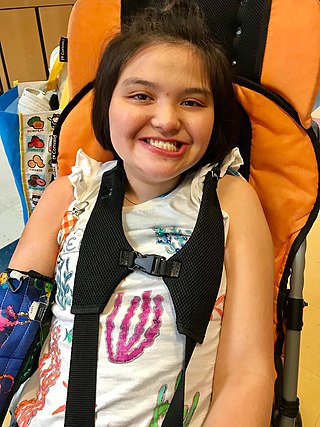
Rett syndrome (RTT) is a genetic disorder that typically becomes apparent after 6–18 months of age and almost exclusively in females. Symptoms include impairments in language and coordination, and repetitive movements. Those affected often have slower growth, difficulty walking, and a smaller head size. Complications of Rett syndrome can include seizures, scoliosis, and sleeping problems. The severity of the condition is variable.

In genetics, a deletion is a mutation in which a part of a chromosome or a sequence of DNA is left out during DNA replication. Any number of nucleotides can be deleted, from a single base to an entire piece of chromosome. Some chromosomes have fragile spots where breaks occur which result in the deletion of a part of chromosome. The breaks can be induced by heat, viruses, radiations, chemicals. When a chromosome breaks, a part of it is deleted or lost, the missing piece of chromosome is referred to as deletion or a deficiency.
Genetics, a discipline of biology, is the science of heredity and variation in living organisms.

Chromosome 15 is one of the 23 pairs of chromosomes in humans. People normally have two copies of this chromosome. Chromosome 15 spans about 99.7 million base pairs and represents between 3% and 3.5% of the total DNA in cells. Chromosome 15 is an acrocentric chromosome, with a very small short arm, which contains few protein coding genes among its 19 million base pairs. It has a larger long arm that is gene rich, spanning about 83 million base pairs.

Medical genetics is the branch of medicine that involves the diagnosis and management of hereditary disorders. Medical genetics differs from human genetics in that human genetics is a field of scientific research that may or may not apply to medicine, while medical genetics refers to the application of genetics to medical care. For example, research on the causes and inheritance of genetic disorders would be considered within both human genetics and medical genetics, while the diagnosis, management, and counselling people with genetic disorders would be considered part of medical genetics.

Dame Kay Elizabeth Davies is a British geneticist. She is Dr Lee's Professor of Anatomy at the University of Oxford and a Fellow of Hertford College, Oxford. She is director of the Medical Research Council (MRC) functional genetics unit, a governor of the Wellcome Trust, a director of the Oxford Centre for Gene Function, and a patron and Senior Member of Oxford University Scientific Society. Her research group has an international reputation for work on Duchenne muscular dystrophy (DMD). In the 1980s, she developed a test which allowed for the screening of foetuses whose mothers have a high risk of carrying DMD.

Gamma-aminobutyric acid receptor subunit beta-3 is a protein that in humans is encoded by the GABRB3 gene. It is located within the 15q12 region in the human genome and spans 250kb. This gene includes 10 exons within its coding region. Due to alternative splicing, the gene codes for many protein isoforms, all being subunits in the GABAA receptor, a ligand-gated ion channel. The beta-3 subunit is expressed at different levels within the cerebral cortex, hippocampus, cerebellum, thalamus, olivary body and piriform cortex of the brain at different points of development and maturity. GABRB3 deficiencies are implicated in many human neurodevelopmental disorders and syndromes such as Angelman syndrome, Prader-Willi syndrome, nonsyndromic orofacial clefts, epilepsy and autism. The effects of methaqualone and etomidate are mediated through GABBR3 positive allosteric modulation.
Barbara J. Meyer is a biologist and genetist, noted for her pioneering research on lambda phage, a virus that infects bacteria; discovery of the master control gene involved in sex determination; and studies of gene regulation, particularly dosage compensation. Meyer's work has revealed mechanisms of sex determination and dosage compensation—that balance X-chromosome gene expression between the sexes in Caenorhabditis elegans that continue to serve as the foundation of diverse areas of study on chromosome structure and function today.
Veronica van Heyningen is an English geneticist who specialises in the etiology of anophthalmia as an honorary professor at University College London (UCL). She previously served as head of medical genetics at the MRC Human Genetics Unit in Edinburgh and the president of The Genetics Society. In 2014 she became president of the Galton Institute. As of 2019 she chairs the diversity committee of the Royal Society, previously chaired by Uta Frith.

Huda Yahya Zoghbi, born Huda El-Hibri, is a Lebanese-born American geneticist, and a professor at the Departments of Molecular and Human Genetics, Neuroscience and Neurology at the Baylor College of Medicine. She is the director of the Jan and Dan Duncan Neurological Research Institute. She became the editor of the Annual Review of Neuroscience as of 2018.
Cognitive genomics is the sub-field of genomics pertaining to cognitive function in which the genes and non-coding sequences of an organism's genome related to the health and activity of the brain are studied. By applying comparative genomics, the genomes of multiple species are compared in order to identify genetic and phenotypical differences between species. Observed phenotypical characteristics related to the neurological function include behavior, personality, neuroanatomy, and neuropathology. The theory behind cognitive genomics is based on elements of genetics, evolutionary biology, molecular biology, cognitive psychology, behavioral psychology, and neurophysiology.

UBE3A-ATS/Ube3a-ATS (human/mouse), otherwise known as ubiquitin ligase E3A-ATS, is the name for the antisense DNA strand that is transcribed as part of a larger transcript called LNCAT at the Ube3a locus. The Ube3a locus is imprinted and in the central nervous system expressed only from the maternal allele. Silencing of Ube3a on the paternal allele is thought to occur through the Ube3a-ATS part of LNCAT, since non-coding antisense transcripts are often found at imprinted loci. The deletion and/or mutation of Ube3a on the maternal chromosome causes Angelman Syndrome (AS) and Ube3a-ATS may prove to be an important aspect in finding a therapy for this disease. While in patients with AS the maternal Ube3a allele is inactive, the paternal allele is intact but epigenetically silenced. If unsilenced, the paternal allele could be a source of active Ube3a protein in AS patients. Therefore, understanding the mechanisms of how Ube3a-ATS might be involved in silencing the paternal Ube3a may lead to new therapies for AS. This possibility has been demonstrated by a recent study where the drug topotecan, administered to mice suffering from AS, activated expression of the paternal Ube3a gene by lowering the transcription of Ube3a-ATS.
Autism spectrum disorder (ASD) refers to a variety of conditions typically identified by challenges with social skills, communication, speech, and repetitive sensory-motor behaviors. The 11th International Classification of Diseases (ICD-11), released in January 2021, characterizes ASD by the associated deficits in the ability to initiate and sustain two-way social communication and restricted or repetitive behavior unusual for the individual's age or situation. Although linked with early childhood, the symptoms can appear later as well. Symptoms can be detected before the age of two and experienced practitioners can give a reliable diagnosis by that age. However, official diagnosis may not occur until much older, even well into adulthood. There is a large degree of variation in how much support a person with ASD needs in day-to-day life. This can be classified by a further diagnosis of ASD level 1, level 2, or level 3. Of these, ASD level 3 describes people requiring very substantial support and who experience more severe symptoms. ASD-related deficits in nonverbal and verbal social skills can result in impediments in personal, family, social, educational, and occupational situations. This disorder tends to have a strong correlation with genetics along with other factors. More research is identifying ways in which epigenetics is linked to autism. Epigenetics generally refers to the ways in which chromatin structure is altered to affect gene expression. Mechanisms such as cytosine regulation and post-translational modifications of histones. Of the 215 genes contributing, to some extent in ASD, 42 have been found to be involved in epigenetic modification of gene expression. Some examples of ASD signs are specific or repeated behaviors, enhanced sensitivity to materials, being upset by changes in routine, appearing to show reduced interest in others, avoiding eye contact and limitations in social situations, as well as verbal communication. When social interaction becomes more important, some whose condition might have been overlooked suffer social and other exclusion and are more likely to have coexisting mental and physical conditions. Long-term problems include difficulties in daily living such as managing schedules, hypersensitivities, initiating and sustaining relationships, and maintaining jobs.
A microdeletion syndrome is a syndrome caused by a chromosomal deletion smaller than 5 million base pairs spanning several genes that is too small to be detected by conventional cytogenetic methods or high resolution karyotyping. Detection is done by fluorescence in situ hybridization (FISH). Larger chromosomal deletion syndromes are detectable using karyotyping techniques.
Dorothy Pamela (DeMontmerency) Warburton was a Canadian geneticist whose research focused on fetal chromosomal abnormalities and reasons for miscarriage. She died at the age of 80 on 26 April 2016 at her home in Englewood, New Jersey.
Elizabeth Mary Claire Fisher is a British geneticist and Professor at University College London. Her research investigates the degeneration of motor neurons during amyotrophic lateral sclerosis and Alzheimer's disease triggered by Down syndrome.
Monica J. Justice is an American–Canadian developmental geneticist. She is the Canada Research Chair in Mammalian Molecular Genetics at the University of Toronto and Program Head of Genetics and Genome Biology at SickKids Hospital.
Elaine H. Zackai is a Professor of Pediatrics, Director of Clinical Genetics, and the Director of the Clinical Genetics Center at Children's Hospital of Philadelphia (CHOP).
References
- ↑ "23andMe's Uta Francke Lauded by ASHG". 23andMe Blog. 2012-11-09. Retrieved 2018-05-25.
- ↑ "Uta Francke's Profile". profiles.stanford.edu. Retrieved 2018-05-25.
- 1 2 3 Azvolinsky, Anna (2018-05-01). "Rare Disease Geneticist: A Profile of Uta Francke". The Scientist Magazine®. Retrieved 2019-12-16.
{{cite web}}: CS1 maint: url-status (link) - ↑ "Uta Francke, MD". HHMI.org. Retrieved 2018-05-25.
- ↑ "Uta Francke's Publications". profiles.stanford.edu. Retrieved 2018-05-25.
- ↑ "Past Recipients". Association for Molecular Pathology. Retrieved 2023-04-12.