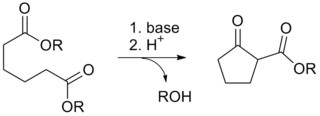In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group attached to an R-group. The general formula of a carboxylic acid is often written as R−COOH or R−CO2H, sometimes as R−C(O)OH with R referring to an organyl group, or hydrogen, or other groups. Carboxylic acids occur widely. Important examples include the amino acids and fatty acids. Deprotonation of a carboxylic acid gives a carboxylate anion.

In chemistry, an ester is a functional group derived from an acid in which the hydrogen atom (H) of at least one acidic hydroxyl group of that acid is replaced by an organyl group. Analogues derived from oxygen replaced by other chalcogens belong to the ester category as well. According to some authors, organyl derivatives of acidic hydrogen of other acids are esters as well, but not according to the IUPAC.

In organic chemistry, a ketone is an organic compound with the structure R−C(=O)−R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group −C(=O)−. The simplest ketone is acetone, with the formula (CH3)2CO. Many ketones are of great importance in biology and industry. Examples include many sugars (ketoses), many steroids, and the solvent acetone.

In organic chemistry, a ketene is an organic compound of the form RR'C=C=O, where R and R' are two arbitrary monovalent chemical groups. The name may also refer to the specific compound ethenone H2C=C=O, the simplest ketene.

In organic chemistry, an aldehyde is an organic compound containing a functional group with the structure R−CH=O. The functional group itself can be referred to as an aldehyde but can also be classified as a formyl group. Aldehydes are a common motif in many chemicals important in technology and biology.
In organic chemistry, a nitrile is any organic compound that has a −C≡N functional group. The name of the compound is composed of a base, which includes the carbon of the −C≡N, suffixed with "nitrile", so for example CH3CH2C≡N is called "propionitrile". The prefix cyano- is used interchangeably with the term nitrile in industrial literature. Nitriles are found in many useful compounds, including methyl cyanoacrylate, used in super glue, and nitrile rubber, a nitrile-containing polymer used in latex-free laboratory and medical gloves. Nitrile rubber is also widely used as automotive and other seals since it is resistant to fuels and oils. Organic compounds containing multiple nitrile groups are known as cyanocarbons.
The Cannizzaro reaction, named after its discoverer Stanislao Cannizzaro, is a chemical reaction which involves the base-induced disproportionation of two molecules of a non-enolizable aldehyde to give a primary alcohol and a carboxylic acid.
In organic chemistry, hydrocyanation is a process for conversion of alkenes to nitriles. The reaction involves the addition of hydrogen cyanide and requires a catalyst. This conversion is conducted on an industrial scale for the production of precursors to nylon.
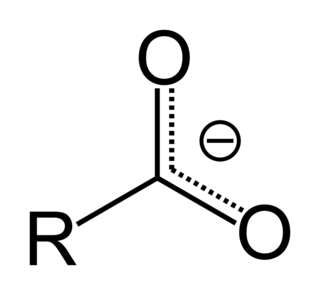
In organic chemistry, a carboxylate is the conjugate base of a carboxylic acid, RCOO−. It is an anion, an ion with negative charge.

The Knorr pyrrole synthesis is a widely used chemical reaction that synthesizes substituted pyrroles (3). The method involves the reaction of an α-amino-ketone (1) and a compound containing an electron-withdrawing group α to a carbonyl group (2).

The Favorskii rearrangement is principally a rearrangement of cyclopropanones and α-halo ketones that leads to carboxylic acid derivatives. In the case of cyclic α-halo ketones, the Favorskii rearrangement constitutes a ring contraction. This rearrangement takes place in the presence of a base, sometimes hydroxide, to yield a carboxylic acid, but usually either an alkoxide base or an amine to yield an ester or an amide, respectively. α,α'-Dihaloketones eliminate HX under the reaction conditions to give α,β-unsaturated carbonyl compounds. Note that trihalomethyl ketone substrates will result in haloform and carboxylate formation via the haloform reaction instead.
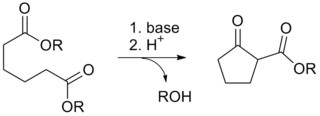
The Dieckmann condensation is the intramolecular chemical reaction of diesters with base to give β-keto esters. It is named after the German chemist Walter Dieckmann (1869–1925). The equivalent intermolecular reaction is the Claisen condensation. Dieckmann condensations are highly effective routes to 5-, 6-, and 7-member rings, but poor for larger rings.
The Hunsdiecker reaction is a name reaction in organic chemistry whereby silver salts of carboxylic acids react with a halogen to produce an organic halide. It is an example of both a decarboxylation and a halogenation reaction as the product has one fewer carbon atoms than the starting material and a halogen atom is introduced its place. A catalytic approach has been developed.

In organic chemistry, an ortho ester is a functional group containing three alkoxy groups attached to one carbon atom, i.e. with the general formula RC(OR<span class="nowrap" style="padding−left:0.05em;">′)3. Orthoesters may be considered as products of exhaustive alkylation of unstable orthocarboxylic acids and it is from these that the name 'ortho ester' is derived. An example is ethyl orthoacetate, CH3C(OCH2CH3)3, more correctly known as 1,1,1-triethoxyethane.
Weerman degradation, also named Weerman reaction, is a name reaction in organic chemistry. It is named after Rudolf Adrian Weerman, who discovered it in 1910. In general, it is an organic reaction in carbohydrate chemistry in which amides are degraded by sodium hypochlorite, forming an aldehyde with one less carbon. Some have regarded it as an extension of the Hofmann rearrangement.
In organic chemistry, a homologation reaction, also known as homologization, is any chemical reaction that converts the reactant into the next member of the homologous series. A homologous series is a group of compounds that differ by a constant unit, generally a methylene group. The reactants undergo a homologation when the number of a repeated structural unit in the molecules is increased. The most common homologation reactions increase the number of methylene units in saturated chain within the molecule. For example, the reaction of aldehydes or ketones with diazomethane or methoxymethylenetriphenylphosphine to give the next homologue in the series.
Selenoxide elimination is a method for the chemical synthesis of alkenes from selenoxides. It is most commonly used to synthesize α,β-unsaturated carbonyl compounds from the corresponding saturated analogues. It is mechanistically related to the Cope reaction.
Alcohol oxidation is a collection of oxidation reactions in organic chemistry that convert alcohols to aldehydes, ketones, carboxylic acids, and esters where the carbon carries a higher oxidation state. The reaction mainly applies to primary and secondary alcohols. Secondary alcohols form ketones, while primary alcohols form aldehydes or carboxylic acids.
The Pinnick oxidation is an organic reaction by which aldehydes can be oxidized into their corresponding carboxylic acids using sodium chlorite (NaClO2) under mild acidic conditions. It was originally developed by Lindgren and Nilsson. The typical reaction conditions used today were developed by G. A. Kraus. H.W. Pinnick later demonstrated that these conditions could be applied to oxidize α,β-unsaturated aldehydes. There exist many different reactions to oxidize aldehydes, but only a few are amenable to a broad range of functional groups. The Pinnick oxidation has proven to be both tolerant of sensitive functionalities and capable of reacting with sterically hindered groups. This reaction is especially useful for oxidizing α,β-unsaturated aldehydes, and another one of its advantages is its relatively low cost.

α,β-Unsaturated carbonyl compounds are organic compounds with the general structure (O=CR)−Cα=Cβ-R. Such compounds include enones and enals, but also carboxylic acids and the corresponding esters and amides. In these compounds, the carbonyl group is conjugated with an alkene. Unlike the case for carbonyls without a flanking alkene group, α,β-unsaturated carbonyl compounds are susceptible to attack by nucleophiles at the β-carbon. This pattern of reactivity is called vinylogous. Examples of unsaturated carbonyls are acrolein (propenal), mesityl oxide, acrylic acid, and maleic acid. Unsaturated carbonyls can be prepared in the laboratory in an aldol reaction and in the Perkin reaction.