In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or in the terminal position. Terminal alkenes are also known as α-olefins.

In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and no other functional groups form a homologous series with the general chemical formula CnH2n−2. Alkynes are traditionally known as acetylenes, although the name acetylene also refers specifically to C2H2, known formally as ethyne using IUPAC nomenclature. Like other hydrocarbons, alkynes are generally hydrophobic.
Cyclobutadiene is an organic compound with the formula C4H4. It is very reactive owing to its tendency to dimerize. Although the parent compound has not been isolated, some substituted derivatives are robust and a single molecule of cyclobutadiene is quite stable. Since the compound degrades by a bimolecular process, the species can be observed by matrix isolation techniques at temperatures below 35 K. It is thought to adopt a rectangular structure.

Vinyl alcohol, also called ethenol or ethylenol, is the simplest enol. With the formula CH2CHOH, it is a labile compound that converts to acetaldehyde immediately upon isolation near room temperature. It is not a practical precursor to any compound.

HU-210 is a synthetic cannabinoid that was first synthesized in 1988 from (1R,5S)-myrtenol by a group led by Raphael Mechoulam at the Hebrew University. HU-210 is 100 to 800 times more potent than natural THC from cannabis and has an extended duration of action. HU-210 has a binding affinity of 0.061 nM at CB1 and 0.52 nM at CB2 in cloned human cannabinoid receptors compared to delta-9-THC of 40.7 nM at CB1. HU-210 is the (–)-1,1-dimethylheptyl analog of 11-hydroxy- Δ8- tetrahydrocannabinol; in some references it is called 1,1-dimethylheptyl- 11-hydroxytetrahydrocannabinol. The abbreviation "HU" stands for Hebrew University.

Dicyclopentadiene, abbreviated DCPD, is a chemical compound with formula C10H12. At room temperature, it is a white brittle wax, although lower purity samples can be straw coloured liquids. The pure material smells somewhat of soy wax or camphor, with less pure samples possessing a stronger acrid odor. Its energy density is 10,975 Wh/l. Dicyclopentadiene is a co-produced in large quantities in the steam cracking of naphtha and gas oils to ethylene. The major use is in resins, particularly, unsaturated polyester resins. It is also used in inks, adhesives, and paints.

In organic chemistry, olefin metathesis is an organic reaction that entails the redistribution of fragments of alkenes (olefins) by the scission and regeneration of carbon-carbon double bonds. Because of the relative simplicity of olefin metathesis, it often creates fewer undesired by-products and hazardous wastes than alternative organic reactions. For their elucidation of the reaction mechanism and their discovery of a variety of highly active catalysts, Yves Chauvin, Robert H. Grubbs, and Richard R. Schrock were collectively awarded the 2005 Nobel Prize in Chemistry.
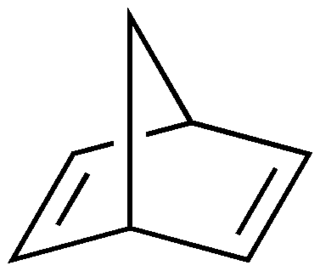
Norbornadiene is an organic compound and a bicyclic hydrocarbon. Norbornadiene is of interest as a metal-binding ligand, whose complexes are useful for homogeneous catalysis. It has been intensively studied owing to its high reactivity and distinctive structural property of being a diene that cannot isomerize. Norbornadiene is also a useful dienophile in Diels-Alder reactions.

(E)-Stilbene, commonly known as trans-stilbene, is an organic compound represented by the condensed structural formula C6H5CH=CHC6H5. Classified as a diarylethene, it features a central ethylene moiety with one phenyl group substituent on each end of the carbon–carbon double bond. It has an (E) stereochemistry, meaning that the phenyl groups are located on opposite sides of the double bond, the opposite of its geometric isomer, cis-stilbene. Trans-stilbene occurs as a white crystalline solid at room temperature and is highly soluble in organic solvents. It can be converted to cis-stilbene photochemically, and further reacted to produce phenanthrene.
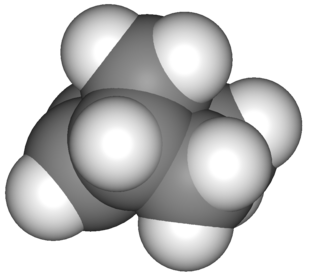
Norbornene or norbornylene or norcamphene is a highly strained bridged cyclic hydrocarbon. It is a white solid with a pungent sour odor. The molecule consists of a cyclohexene ring with a methylene bridge between carbons 1 and 4. The molecule carries a double bond which induces significant ring strain and significant reactivity.
A triazole is a heterocyclic compound featuring a five-membered ring of two carbon atoms and three nitrogen atoms with molecular formula C2H3N3. Triazoles exhibit substantial isomerism, depending on the positioning of the nitrogen atoms within the ring.

In organic chemistry, an anti-Bredt molecule is a bridged molecule with a double bond at the bridgehead. Bredt's rule is the empirical observation that such molecules only form in large enough ring systems. For example, two of the following norbornene isomers violate Bredt's rule, and are too unstable to prepare:
1,2-Dichloroethylene or 1,2-DCE is the name for a pair of organochlorine compounds with the molecular formula C2H2Cl2. The two compounds are isomers, each being colorless liquids with a sweet odor. It can exist as either of two geometric isomers, cis-1,2-dichloroethene or trans-1,2-dichloroethene, but is often used as a mixture of the two. They have modest solubility in water. These compounds have some applications as a degreasing solvent. In contrast to most cis-trans compounds, the Z isomer (cis) is more stable than the E isomer (trans) by 0.4 kcal/mol.

Methyl vinyl ketone (MVK, IUPAC name: butenone) is the organic compound with the formula CH3C(O)CH=CH2. It is a reactive compound classified as an enone, in fact the simplest example thereof. It is a colorless, flammable, highly toxic liquid with a pungent odor. It is soluble in water and polar organic solvents. It is a useful intermediate in the synthesis of other compounds.
In polymer chemistry, ring-opening metathesis polymerization (ROMP) is a type of chain-growth polymerization involving olefin metathesis. The driving force of the reaction is relief of ring strain in cyclic olefins. A variety of heterogeneous and homogeneous catalysts have been developed. Most large-scale commercial processes rely on the former while some fine chemical syntheses rely on the homogeneous catalysts. Catalysts are based on transition metals such as tungsten, molybdenum, rhenium, rubidium, and titanium.

Dewar benzene (also spelled dewarbenzene) or bicyclo[2.2.0]hexa-2,5-diene is a bicyclic isomer of benzene with the molecular formula C6H6. The compound is named after James Dewar who included this structure in a list of possible C6H6 structures in 1869. However, he did not propose it as the structure of benzene, and in fact he supported the correct structure previously proposed by August Kekulé in 1865.
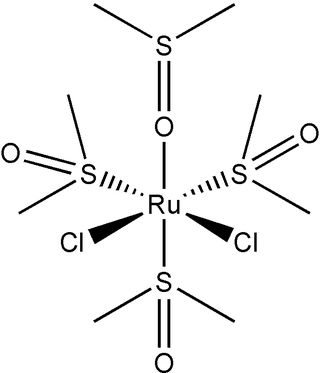
Dichlorotetrakis(dimethyl sulfoxide) ruthenium(II) describes coordination compounds with the formula RuCl2(dmso)4, where DMSO is dimethylsulfoxide. Both cis and trans isomers are known, but the cis isomer is more common. The cis isomer is a yellow, air-stable solid that is soluble in some organic solvents. These sulfoxide complexes are used in the synthesis of various ruthenium(ii) complexes. They have also attracted attention as possible anti-cancer drugs.

Norbormide is a toxic compound used as a rodenticide. It has several mechanisms of action, acting as a vasoconstrictor and calcium channel blocker, but is selectively toxic to rats and has relatively low toxicity to other species, due to a species specific action of opening the permeability transition pores in rat mitochondria.

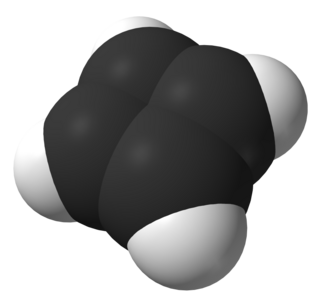
Ethylidene norbornene (ENB) is an organic compound that consists of an ethylidene (CH3C(H)=) group attached to norbornene. It is a colorless liquid. The molecule consists of two sites of unsaturation. The compound consists of E- and Z-stereoisomers, but the mixtures are typically not separated.

Nadic anhydride, also known as 5-norbornene-2,3-dicarboxylic anhydride, is an organic acid anhydride derivative of norbornene.