An endosymbiont or endobiont is any organism that lives within the body or cells of another organism most often, though not always, in a mutualistic relationship. (The term endosymbiosis is from the Greek: ἔνδον endon "within", σύν syn "together" and βίωσις biosis "living".) Examples are nitrogen-fixing bacteria, which live in the root nodules of legumes, single-cell algae inside reef-building corals and bacterial endosymbionts that provide essential nutrients to insects.
Peptides are short chains of amino acids linked by peptide bonds. A polypeptide is a longer, continuous, unbranched peptide chain. Polypeptides which have a molecular mass of 10,000 Da or more are called proteins. Chains of fewer than twenty amino acids are called oligopeptides, and include dipeptides, tripeptides, and tetrapeptides.

An endophyte is an endosymbiont, often a bacterium or fungus, that lives within a plant for at least part of its life cycle without causing apparent disease. Endophytes are ubiquitous and have been found in all species of plants studied to date; however, most of the endophyte/plant relationships are not well understood. Some endophytes may enhance host growth, nutrient acquisition and improve the plant's ability to tolerate abiotic stresses, such as drought and decrease biotic stresses by enhancing plant resistance to insects, pathogens and herbivores. Although endophytic bacteria and fungi are frequently studied, endophytic archaea are increasingly being considered for their role in plant growth promotion as part of the core microbiome of a plant.
Edman degradation, developed by Pehr Edman, is a method of sequencing amino acids in a peptide. In this method, the amino-terminal residue is labeled and cleaved from the peptide without disrupting the peptide bonds between other amino acid residues.
Nonribosomal peptides (NRP) are a class of peptide secondary metabolites, usually produced by microorganisms like bacteria and fungi. Nonribosomal peptides are also found in higher organisms, such as nudibranchs, but are thought to be made by bacteria inside these organisms. While there exist a wide range of peptides that are not synthesized by ribosomes, the term nonribosomal peptide typically refers to a very specific set of these as discussed in this article.

Antimicrobial peptides (AMPs), also called host defence peptides (HDPs) are part of the innate immune response found among all classes of life. Fundamental differences exist between prokaryotic and eukaryotic cells that may represent targets for antimicrobial peptides. These peptides are potent, broad spectrum antibiotics which demonstrate potential as novel therapeutic agents. Antimicrobial peptides have been demonstrated to kill Gram negative and Gram positive bacteria, enveloped viruses, fungi and even transformed or cancerous cells. Unlike the majority of conventional antibiotics it appears that antimicrobial peptides frequently destabilize biological membranes, can form transmembrane channels, and may also have the ability to enhance immunity by functioning as immunomodulators.

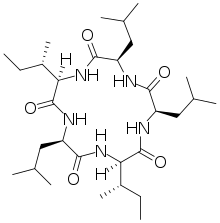
Gramicidin S or Gramicidin Soviet is an antibiotic that is effective against some gram-positive and gram-negative bacteria as well as some fungi.

Tyrocidine is a mixture of cyclic decapeptides produced by the bacteria Bacillus brevis found in soil. It can be composed of 4 different amino acid sequences, giving tyrocidine A–D. Tyrocidine is the major constituent of tyrothricin, which also contains gramicidin. Tyrocidine was the first commercially available antibiotic, but has been found to be toxic toward human blood and reproductive cells. The function of tyrocidine within its host B. brevis is thought to be regulation of sporulation.

Cyclic peptides are polypeptide chains which contain a circular sequence of bonds. This can be through a connection between the amino and carboxyl ends of the peptide, for example in cyclosporin; a connection between the amino end and a side chain, for example in bacitracin; the carboxyl end and a side chain, for example in colistin; or two side chains or more complicated arrangements, for example in amanitin. Many cyclic peptides have been discovered in nature and many others have been synthesized in the laboratory. Their length ranges from just two amino acid residues to hundreds. In nature they are frequently antimicrobial or toxic; in medicine they have various applications, for example as antibiotics and immunosuppressive agents. Thin-Layer Chromatography (TLC) is a convenient method to detect cyclic peptides in crude extract from bio-mass.
A tetrapeptide is a peptide, classified as an oligopeptide, since it only consists of four amino acids joined by peptide bonds. Many tetrapeptides are pharmacologically active, often showing affinity and specificity for a variety of receptors in protein-protein signaling. Present in nature are both linear and cyclic tetrapeptides (CTPs), the latter of which mimics protein reverse turns which are often present on the surface of proteins and druggable targets. Tetrapeptides may be cyclized by a fourth peptide bond or other covalent bonds.

Cyclochlorotine (CC), also known as islanditoxin is a mycotoxin produced by the fungus Penicillium islandicum that causes liver damage and has carcinogenic properties. Japanese researchers confirmed that it was one of three strains of Penicillin fungi responsible for yellowed rice. It is listed as an IARC Group 3 carcinogen.
Theta-defensins are a family of mammalian antimicrobial peptides. They are found in non-human 'Old World' primates, but not in human, gorilla, bonobo, and chimpanzee.
Plant use of endophytic fungi in defense occurs when endophytic fungi, which live symbiotically with the majority of plants by entering their cells, are utilized as an indirect defense against herbivores. In exchange for carbohydrate energy resources, the fungus provides benefits to the plant which can include increased water or nutrient uptake and protection from phytophagous insects, birds or mammals. Once associated, the fungi alter nutrient content of the plant and enhance or begin production of secondary metabolites. The change in chemical composition acts to deter herbivory by insects, grazing by ungulates and/or oviposition by adult insects. Endophyte-mediated defense can also be effective against pathogens and non-herbivory damage.

Marine fungi are species of fungi that live in marine or estuarine environments. They are not a taxonomic group, but share a common habitat. Obligate marine fungi grow exclusively in the marine habitat while wholly or sporadically submerged in sea water. Facultative marine fungi normally occupy terrestrial or freshwater habitats, but are capable of living or even sporulating in a marine habitat. About 444 species of marine fungi have been described, including seven genera and ten species of basidiomycetes, and 177 genera and 360 species of ascomycetes. The remainder of the marine fungi are chytrids and mitosporic or asexual fungi. Many species of marine fungi are known only from spores and it is likely a large number of species have yet to be discovered. In fact, it is thought that less than 1% of all marine fungal species have been described, due to difficulty in targeting marine fungal DNA and difficulties that arise in attempting to grow cultures of marine fungi. It is impracticable to culture many of these fungi, but their nature can be investigated by examining seawater samples and undertaking rDNA analysis of the fungal material found.
Scott A. Strobel is the provost, Henry Ford II professor of molecular biophysics and biochemistry, and a professor of chemistry at Yale University. He was the vice provost for Science Initiatives and vice president for West Campus Planning & Program Development. An educator and researcher, he has led a number of Yale initiatives over the past two decades. Strobel was appointed as Yale's provost in 2020.
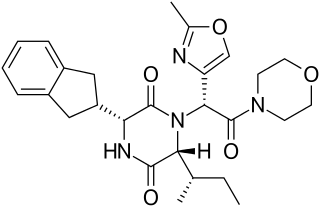
A diketopiperazine (DKP), also known as a dioxopiperazine or piperazinedione, is a class of organic compounds related to piperazine but containing two amide linkages. DKP's are the smallest known class of cyclic peptide. Despite their name, they are not ketones, but amides. Three regioisomers are possible, differing in the locations of the carbonyl groups.
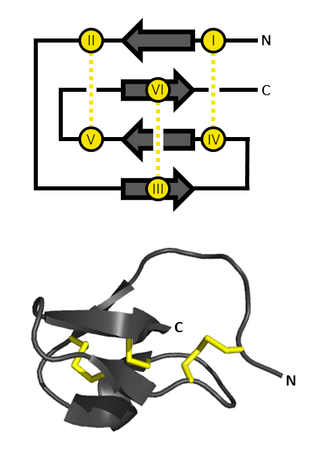
An inhibitor cystine knot is a protein structural motif containing three disulfide bridges. Knottins are one of three folds in the cystine knot motif; the other closely related knots are the Growth Factor Cystine Knot (GFCK) and the Cyclic Cystine Knot. Types include a) cyclic mobius, b) cyclic bracelet, c) acyclic inhibitor knottins. Cystine knot motifs are found frequently in nature in a plethora of plants, animals, and fungi and serve diverse functions from appetite suppression to anti-fungal activity.

Myrcia guianensis (pedra-ume-caá) is a species of plant in the genus Myrcia of the family Myrtaceae native to South America.

The phomoxanthones are a loosely defined class of natural products. The two founding members of this class are phomoxanthone A and phomoxanthone B. Other compounds were later also classified as phomoxanthones, although a unifying nomenclature has not yet been established. The structure of all phomoxanthones is derived from a dimer of two covalently linked tetrahydroxanthones, and they differ mainly in the position of this link as well as in the acetylation status of their hydroxy groups. The phomoxanthones are structurally closely related to other tetrahydroxanthone dimers such as the secalonic acids and the eumitrins. While most phomoxanthones were discovered in fungi of the genus Phomopsis, most notably in the species Phomopsis longicolla, some have also been found in Penicillium sp.