
A sugar substitute is a food additive that provides a sweetness like that of sugar while containing significantly less food energy than sugar-based sweeteners, making it a zero-calorie or low-calorie sweetener. Artificial sweeteners may be derived through manufacturing of plant extracts or processed by chemical synthesis. Sugar substitute products are commercially available in various forms, such as small pills, powders, and packets.

Xylitol is a chemical compound with the formula C
5H
12O
5, or HO(CH2)(CHOH)3(CH2)OH; specifically, one particular stereoisomer with that structural formula. It is a colorless or white crystalline solid that is freely soluble in water. It can be classified as a polyalcohol and a sugar alcohol, specifically an alditol. The name derives from Ancient Greek: ξύλον, xyl[on] 'wood', with the suffix -itol used to denote sugar alcohols.

Chewing gum is a soft, cohesive substance designed to be chewed without being swallowed. Modern chewing gum is composed of gum base, sweeteners, softeners/plasticizers, flavors, colors, and, typically, a hard or powdered polyol coating. Its texture is reminiscent of rubber because of the physical-chemical properties of its polymer, plasticizer, and resin components, which contribute to its elastic-plastic, sticky, chewy characteristics.
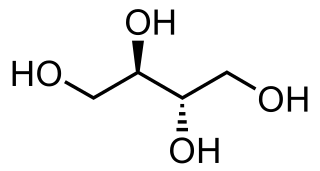
Sugar alcohols are organic compounds, typically derived from sugars, containing one hydroxyl group (−OH) attached to each carbon atom. They are white, water-soluble solids that can occur naturally or be produced industrially by hydrogenating sugars. Since they contain multiple −OH groups, they are classified as polyols.

A polycyclic aromatic hydrocarbon (PAH) is a class of organic compounds that is composed of multiple aromatic rings. The simplest representative is naphthalene, having two aromatic rings, and the three-ring compounds anthracene and phenanthrene. PAHs are uncharged, non-polar and planar. Many are colorless. Many of them are found in coal and in oil deposits, and are also produced by the incomplete combustion of organic matter—for example, in engines and incinerators or when biomass burns in forest fires.

Erythritol (, ) is an organic compound, the naturally occurring achiral meso four-carbon sugar alcohol (or polyol). It is the reduced form of either D- or L-erythrose and one of the two reduced forms of erythrulose. It is used as a food additive and sugar substitute. It is synthesized from corn using enzymes and fermentation. Its formula is C
4H
10O
4, or HO(CH2)(CHOH)2(CH2)OH.
Volatile organic compounds (VOCs) are organic compounds that have a high vapor pressure at room temperature. High vapor pressure correlates with a low boiling point, which relates to the number of the sample's molecules in the surrounding air, a trait known as volatility.

Cerium(III) chloride (CeCl3), also known as cerous chloride or cerium trichloride, is a compound of cerium and chlorine. It is a white hygroscopic salt; it rapidly absorbs water on exposure to moist air to form a hydrate, which appears to be of variable composition, though the heptahydrate CeCl3·7H2O is known. It is highly soluble in water, and (when anhydrous) it is soluble in ethanol and acetone.
Organic synthesis is a branch of chemical synthesis concerned with the construction of organic compounds. Organic compounds are molecules consisting of combinations of covalently-linked hydrogen, carbon, oxygen, and nitrogen atoms. Within the general subject of organic synthesis, there are many different types of synthetic routes that can be completed including total synthesis, stereoselective synthesis, automated synthesis, and many more. Additionally, in understanding organic synthesis it is necessary to be familiar with the methodology, techniques, and applications of the subject.
In organic chemistry, a polyol is an organic compound containing multiple hydroxyl groups. The term "polyol" can have slightly different meanings depending on whether it is used in food science or polymer chemistry. Polyols containing two, three and four hydroxyl groups are diols, triols, and tetrols, respectively.
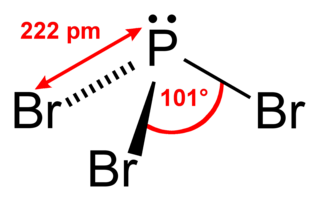
Phosphorus tribromide is a colourless liquid with the formula PBr3. The liquid fumes in moist air due to hydrolysis and has a penetrating odour. It is used in the laboratory for the conversion of alcohols to alkyl bromides.

Streptococcus mutans is a facultatively anaerobic, gram-positive coccus commonly found in the human oral cavity and is a significant contributor to tooth decay. It is part of the "streptococci", an informal general name for all species in the genus Streptococcus. The microbe was first described by James Kilian Clarke in 1924.
The Ullmann reaction or Ullmann coupling, named after Fritz Ullmann, couples two aryl or alkyl groups with the help of copper. The reaction was first reported by Ullmann and his student Bielecki in 1901. It has been later shown that palladium and nickel can also be effectively used.
The Wurtz–Fittig reaction is the chemical reaction of an aryl halide, alkyl halides, and sodium metal to give substituted aromatic compounds. Following the work of Charles Adolphe Wurtz on the sodium-induced coupling of alkyl halides, Wilhelm Rudolph Fittig extended the approach to the coupling of an alkyl halide with an aryl halide. This modification of the Wurtz reaction is considered a separate process and is named for both scientists.

Sir David William Cross MacMillan is a Scottish chemist and the James S. McDonnell Distinguished University Professor of Chemistry at Princeton University, where he was also the chair of the Department of Chemistry from 2010 to 2015. He shared the 2021 Nobel Prize in Chemistry with Benjamin List "for the development of asymmetric organocatalysis". MacMillan used his share of the $1.14 million prize to establish the May and Billy MacMillan Foundation.

Soil carbon is the solid carbon stored in global soils. This includes both soil organic matter and inorganic carbon as carbonate minerals. It is vital to the soil capacity in our ecosystem. Soil carbon is a carbon sink in regard to the global carbon cycle, playing a role in biogeochemistry, climate change mitigation, and constructing global climate models. Natural variation such as organisms and time has affected the management of carbon in the soils. The major influence has been that of human activities which has caused a massive loss of soil organic carbon. An example of human activity includes fire which destroys the top layer of the soil and the soil therefore get exposed to excessive oxidation.

In enzymology, a D-xylulose reductase (EC 1.1.1.9) is an enzyme that is classified as an Oxidoreductase (EC 1) specifically acting on the CH-OH group of donors (EC 1.1.1) that uses NAD+ or NADP+ as an acceptor (EC 1.1.1.9). This enzyme participates in pentose and glucuronate interconversions; a set of metabolic pathways that involve converting pentose sugars and glucuronate into other compounds.

Xylitol pentanitrate (XPN) is a nitrated ester primary explosive first synthesized in 1891 by Gabriel Bertrand. Law enforcement has taken an interest in XPN along with erythritol tetranitrate (ETN) and pentaerythritol tetranitrate (PETN) due to their ease of synthesis, which makes them accessible to amateur chemists and terrorists.

Tooth remineralization is the natural repair process for non-cavitated tooth lesions, in which calcium, phosphate and sometimes fluoride ions are deposited into crystal voids in demineralised enamel. Remineralization can contribute towards restoring strength and function within tooth structure.

Arthrobacter bussei is a pink-coloured, aerobic, coccus-shaped, Gram-stain-positive, oxidase-positive and catalase-positive bacterium isolated from cheese made of cow´s milk. A. bussei is non-motile and does not form spores. Rod–coccus life cycle is not observed. Cells are 1.1–1.5 µm in diameter. On trypticase soy agar it forms pink-coloured, raised and round colonies, which are 1.0 mm in diameter after 5 days at 30 °C The genome of the strain A. bussei KR32T has been fully sequenced.















