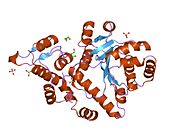
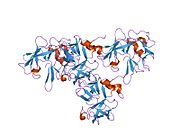
An aminoacyl-tRNA synthetase, also called tRNA-ligase, is an enzyme that attaches the appropriate amino acid onto its corresponding tRNA. It does so by catalyzing the transesterification of a specific cognate amino acid or its precursor to one of all its compatible cognate tRNAs to form an aminoacyl-tRNA. In humans, the 20 different types of aa-tRNA are made by the 20 different aminoacyl-tRNA synthetases, one for each amino acid of the genetic code.
Tyrosine—tRNA ligase, also known as tyrosyl-tRNA synthetase is an enzyme that is encoded by the gene YARS. Tyrosine—tRNA ligase catalyzes the chemical reaction

Tristetraprolin (TTP), also known as zinc finger protein 36 homolog (ZFP36), is a protein that in humans, mice and rats is encoded by the ZFP36 gene. It is a member of the TIS11 family, along with butyrate response factors 1 and 2.
Aminoacyl tRNA synthetase complex-interacting multifunctional protein 1 is a protein that in humans is encoded by the AIMP1 gene.
Elongation factor 1-gamma is a protein that in humans is encoded by the EEF1G gene.
Bifunctional aminoacyl-tRNA synthetase is an enzyme that in humans is encoded by the EPRS gene.

Aspartyl-tRNA synthetase, cytoplasmic is an enzyme that in humans is encoded by the DARS gene.

Leucyl-tRNA synthetase, cytoplasmic is an enzyme that in humans is encoded by the LARS gene.

Glutaminyl-tRNA synthetase is an enzyme that in humans is encoded by the QARS gene.
Methionyl-tRNA synthetase, cytoplasmic is an enzyme that in humans is encoded by the MARS gene.

Threonyl-tRNA synthetase, cytoplasmic is an enzyme that in humans is encoded by the TARS gene.

Isoleucyl-tRNA synthetase, cytoplasmic is an enzyme that in humans is encoded by the IARS1 gene.

Very long-chain acyl-CoA synthetase is an enzyme that in humans is encoded by the SLC27A2 gene.

Zinc finger protein 143 is a protein that in humans is encoded by the ZNF143 gene.

Tryptophanyl-tRNA synthetase, mitochondrial is an enzyme that in humans is encoded by the WARS2 gene.

Phenylalanyl-tRNA synthetase alpha chain is an enzyme that in humans is encoded by the FARSA gene.

Probable histidyl-tRNA synthetase, mitochondrial is an enzyme that in humans is encoded by the HARS2 gene.
Susan A. Martinis is an American biochemist. She has co-authored over 57 publications in peer reviewed journals and scientific book chapters. Her expertise is in protein:RNA interactions and aminoacyl tRNA synthetases. As of 2019, she is the Vice Chancellor for Research and Innovation at the University of Illinois at Urbana-Champaign.
Karin Musier-Forsyth, an American biochemist, is an Ohio Eminent Scholar on the faculty of the Department of Chemistry & Biochemistry at Ohio State University. Musier-Forsyth's research involves biochemical, biophysical and cell-based approaches to understand the interactions of proteins and RNAs involved in protein synthesis and viral replication, especially in HIV.
Xiang-Lei Yang (杨湘磊) is a Chinese-born American molecular biologist. She is a professor at The Scripps Research Institute, located in La Jolla, California. Her work has contributed to the establishment of physiological importance of aminoacyl-tRNA synthetases beyond their classical role in supporting mRNA translation and their disordered processes that contribute to disease. She founded the Translation Machinery in Health and Disease Gordon Research Conference, an ongoing biannual international conference since 2015. She helped co-found aTyr Pharma, a Nasdaq-listed biotechnology company.