
The mevalonate pathway, also known as the isoprenoid pathway or HMG-CoA reductase pathway is an essential metabolic pathway present in eukaryotes, archaea, and some bacteria. The pathway produces two five-carbon building blocks called isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP), which are used to make isoprenoids, a diverse class of over 30,000 biomolecules such as cholesterol, vitamin K, coenzyme Q10, and all steroid hormones.

Dimethylallyl pyrophosphate is an isoprenoid precursor. It is a product of both the mevalonate pathway and the MEP pathway of isoprenoid precursor biosynthesis. It is an isomer of isopentenyl pyrophosphate (IPP) and exists in virtually all life forms. The enzyme isopentenyl pyrophosphate isomerase catalyzes isomerization between DMAPP and IPP.

Isopentenyl pyrophosphate is an isoprenoid precursor. IPP is an intermediate in the classical, HMG-CoA reductase pathway and in the non-mevalonate MEP pathway of isoprenoid precursor biosynthesis. Isoprenoid precursors such as IPP, and its isomer DMAPP, are used by organisms in the biosynthesis of terpenes and terpenoids.
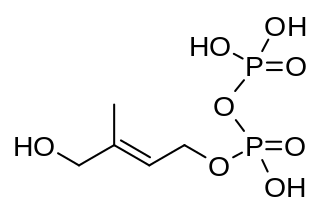
(E)-4-Hydroxy-3-methyl-but-2-enyl pyrophosphate is an intermediate of the MEP pathway of isoprenoid biosynthesis. The enzyme HMB-PP synthase catalyzes the conversion of 2-C-methyl-D-erythritol 2,4-cyclodiphosphate (MEcPP) into HMB-PP. HMB-PP is then converted further to isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP) by HMB-PP reductase.
The non-mevalonate pathway—also appearing as the mevalonate-independent pathway and the 2-C-methyl-D-erythritol 4-phosphate/1-deoxy-D-xylulose 5-phosphate (MEP/DOXP) pathway—is an alternative metabolic pathway for the biosynthesis of the isoprenoid precursors isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP). The currently preferred name for this pathway is the MEP pathway, since MEP is the first committed metabolite on the route to IPP.

Squalene synthase (SQS) or farnesyl-diphosphate:farnesyl-diphosphate farnesyl transferase is an enzyme localized to the membrane of the endoplasmic reticulum. SQS participates in the isoprenoid biosynthetic pathway, catalyzing a two-step reaction in which two identical molecules of farnesyl pyrophosphate (FPP) are converted into squalene, with the consumption of NADPH. Catalysis by SQS is the first committed step in sterol synthesis, since the squalene produced is converted exclusively into various sterols, such as cholesterol, via a complex, multi-step pathway. SQS belongs to squalene/phytoene synthase family of proteins.

DXP reductoisomerase is an enzyme that interconverts 1-deoxy-D-xylulose 5-phosphate (DXP) and 2-C-methyl-D-erythritol 4-phosphate (MEP).

2-C-Methyl-D-erythritol 4-phosphate (MEP) is an intermediate on the MEP pathway of isoprenoid precursor biosynthesis. It is the first committed metabolite on that pathway on the route to IPP and DMAPP.
In enzymology, a 4-hydroxy-3-methylbut-2-en-1-yl diphosphate synthase (HMB-PP synthase, IspG, EC 1.17.7.1) is an enzyme that catalyzes the chemical reaction
2-C-methyl-D-erythritol 2,4-cyclodiphosphate synthase is a zinc-dependent enzyme and a member of the YgbB N terminal protein domain, which participates in the MEP pathway of isoprenoid precursor biosynthesis. It catalyzes the chemical reaction:

Diphosphomevalonate decarboxylase (EC 4.1.1.33), most commonly referred to in scientific literature as mevalonate diphosphate decarboxylase, is an enzyme that catalyzes the chemical reaction

In molecular biology, the protein domain SAICAR synthase is an enzyme which catalyses a reaction to create SAICAR. In enzymology, this enzyme is also known as phosphoribosylaminoimidazolesuccinocarboxamide synthase. It is an enzyme that catalyzes the chemical reaction

In molecular biology, hydroxymethylglutaryl-CoA synthase or HMG-CoA synthase EC 2.3.3.10 is an enzyme which catalyzes the reaction in which acetyl-CoA condenses with acetoacetyl-CoA to form 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA). This reaction comprises the second step in the mevalonate-dependent isoprenoid biosynthesis pathway. HMG-CoA is an intermediate in both cholesterol synthesis and ketogenesis. This reaction is overactivated in patients with diabetes mellitus type 1 if left untreated, due to prolonged insulin deficiency and the exhaustion of substrates for gluconeogenesis and the TCA cycle, notably oxaloacetate. This results in shunting of excess acetyl-CoA into the ketone synthesis pathway via HMG-CoA, leading to the development of diabetic ketoacidosis.

In enzymology, a 2-C-methyl-D-erythritol 4-phosphate cytidylyltransferase is an enzyme that catalyzes the chemical reaction:
In enzymology, a 4-(cytidine 5'-diphospho)-2-C-methyl-D-erythritol kinase is an enzyme that catalyzes the chemical reaction

2-C-Methyl-d-erythritol-2,4-cyclopyrophosphate (MEcPP) is an intermediate in the MEP pathway (non-mevalonate) of isoprenoid precursor biosynthesis. MEcPP is produced by MEcPP synthase (IspF) and is a substrate for HMB-PP synthase (IspG).
4-Diphosphocytidyl-2-C-methylerythritol is an intermediate in the MEP pathway of isoprenoid precursor biosynthesis. It is produced by the enzyme 2-C-methyl-D-erythritol 4-phosphate cytidylyltransferase (IspD) and is a substrate for CDP-ME kinase (IspE).
4-Diphosphocytidyl-2-C-methyl-D-erythritol 2-phosphate is an intermediate in the MEP pathway of isoprenoid precursor biosynthesis.
4-Hydroxy-3-methylbut-2-enyl diphosphate reductase (EC 1.17.1.2, isopentenyl-diphosphate:NADP+ oxidoreductase, LytB, (E)-4-hydroxy-3-methylbut-2-en-1-yl diphosphate reductase, HMBPP reductase, IspH, LytB/IspH) is an enzyme in the non-mevalonate pathway. It acts upon (E)-4-Hydroxy-3-methyl-but-2-enyl pyrophosphate (or "HMB-PP").
Michel Rohmer, born on 31 January 1948, is a French chemist specialising in the chemistry of micro-organisms. He has particularly studied isoprenoids.












