Peptides are short chains of amino acids linked by peptide bonds. A polypeptide is a longer, continuous, unbranched peptide chain. Polypeptides which have a molecular mass of 10,000 Da or more are called proteins. Chains of fewer than twenty amino acids are called oligopeptides, and include dipeptides, tripeptides, and tetrapeptides.

In organic chemistry, a peptide bond is an amide type of covalent chemical bond linking two consecutive alpha-amino acids from C1 of one alpha-amino acid and N2 of another, along a peptide or protein chain.
In molecular biology, elastase is an enzyme from the class of proteases (peptidases) that break down proteins. In particular, it is a serine protease.

Polymyxins are antibiotics. Polymyxins B and E are used in the treatment of Gram-negative bacterial infections. They work mostly by breaking up the bacterial cell membrane. They are part of a broader class of molecules called nonribosomal peptides.
Nonribosomal peptides (NRP) are a class of peptide secondary metabolites, usually produced by microorganisms like bacteria and fungi. Nonribosomal peptides are also found in higher organisms, such as nudibranchs, but are thought to be made by bacteria inside these organisms. While there exist a wide range of peptides that are not synthesized by ribosomes, the term nonribosomal peptide typically refers to a very specific set of these as discussed in this article.

Gramicidin S or Gramicidin Soviet is an antibiotic that is effective against some gram-positive and gram-negative bacteria as well as some fungi.
Cyanogen bromide is the inorganic compound with the formula (CN)Br or BrCN. It is a colorless solid that is widely used to modify biopolymers, fragment proteins and peptides, and synthesize other compounds. The compound is classified as a pseudohalogen.
Melanocortin receptors are members of the rhodopsin family of 7-transmembrane G protein-coupled receptors.

Tyrocidine is a mixture of cyclic decapeptides produced by the bacteria Bacillus brevis found in soil. It can be composed of 4 different amino acid sequences, giving tyrocidine A–D. Tyrocidine is the major constituent of tyrothricin, which also contains gramicidin. Tyrocidine was the first commercially available antibiotic, but has been found to be toxic toward human blood and reproductive cells. The function of tyrocidine within its host B. brevis is thought to be regulation of sporulation.

Cyclic peptides are polypeptide chains which contain a circular sequence of bonds. This can be through a connection between the amino and carboxyl ends of the peptide, for example in cyclosporin; a connection between the amino end and a side chain, for example in bacitracin; the carboxyl end and a side chain, for example in colistin; or two side chains or more complicated arrangements, for example in amanitin. Many cyclic peptides have been discovered in nature and many others have been synthesized in the laboratory. Their length ranges from just two amino acid residues to hundreds. In nature they are frequently antimicrobial or toxic; in medicine they have various applications, for example as antibiotics and immunosuppressive agents. Thin-Layer Chromatography (TLC) is a convenient method to detect cyclic peptides in crude extract from bio-mass.
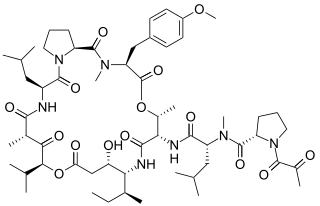
Plitidepsin is a chemical compound extracted from the ascidian Aplidium albicans. It is currently undergoing clinical trial testing. It is a member of the class of compounds known as didemnins.

Surfactin is a cyclic lipopeptide, commonly used as an antibiotic for its capacity as a surfactant. It is an amphiphile capable of withstanding hydrophilic and hydrophobic environments. The Gram-positive bacterial species Bacillus subtilis produces surfactin for its antibiotic effects against competitors. Surfactin showcases antibacterial, antiviral, antifungal, and hemolytic effects.
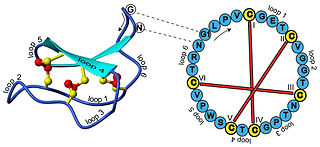
In biochemistry, cyclotides are small, disulfide-rich peptides isolated from plants. Typically containing 28-37 amino acids, they are characterized by their head-to-tail cyclised peptide backbone and the interlocking arrangement of their three disulfide bonds. These combined features have been termed the cyclic cystine knot (CCK) motif. To date, over 100 cyclotides have been isolated and characterized from species of the families Rubiaceae, Violaceae, and Cucurbitaceae. Cyclotides have also been identified in agriculturally important families such as the Fabaceae and Poaceae.

Antamanide is a cyclic decapeptide isolated from a fungus, the death cap: Amanita phalloides. It is being studied as a potential anti-toxin against the effects of phalloidin and for its potential for treating edema. It contains 1 valine residue, 4 proline residues, 1 alanine residue, and 4 phenylalanine residues with a structure of c(Val-Pro-Pro-Ala-Phe-Phe-Pro-Pro-Phe-Phe). It was isolated by determining the source of the anti-phalloidin activity from a lipophillic extraction from the organism. It has been shown that antamanide can react to form alkali metal ion complexes. These include complexes with sodium and calcium ions. When these complexes are formed, the cyclopeptide structure undergoes a conformational change.

Class II bacteriocins are a class of small peptides that inhibit the growth of various bacteria.
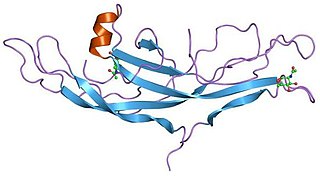
A cystine knot is a protein structural motif containing three disulfide bridges. The sections of polypeptide that occur between two of them form a loop through which a third disulfide bond passes, forming a rotaxane substructure. The cystine knot motif stabilizes protein structure and is conserved in proteins across various species. There are three types of cystine knot, which differ in the topology of the disulfide bonds:
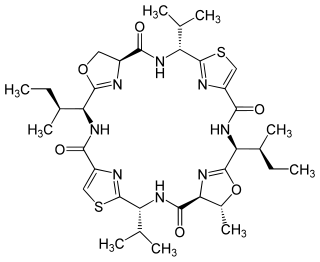
Patellamide A is a peptide natural product produced by Prochloron didemni, a cyanobacterial symbiont of Lissoclinum patella, and was first isolated in 1981. Patellamide A is one of many didemnid peptides. Other closely related peptides include patellamides B, C, and D and trunkamide. The patellamides and trunkamide show moderate cytotoxicity and activity against multidrug resistant cancer cell lines.
Split-intein circular ligation of peptides and proteins (SICLOPPS) is a biotechnology technique that permits the creation of cyclic peptides. These peptides are produced by ribosomal protein synthesis, followed by an intein-like event that splices the protein into a loop. By contrast with the nonribosomal peptide synthetases that produces some cyclic peptides like gramicidin S, SICLOPPS offers the advantage that the peptides' structure can be encoded by DNA in a simple manner according to the genetic code, but for this reason it imposes limitations on the types of amino acids incorporated that are comparable to those that apply to ordinary proteins. As implemented there is also some constraint on the peptide sequence of the cyclic sequence; for example, libraries may use the sequence SGXX..XXPL to increase the efficiency of circularization of the peptide. SICLOPPS is frequently used with a library of randomized DNA sequence that permits the simultaneous production and screening of large numbers of constructs at once, followed by the recovery of the DNA sequences responsible for the activity of the clone of interest.
Virotoxins are monocyclic peptides formed by at least five different compounds: alaviroidin, viroisin, deoxoviroisin, viroidin, and deoxoviroidin. The structure and biological activity of virotoxins are similar to that of phallotoxins, thus suggesting that virotoxins are biosynthetically derived from phallotoxins or share common precursor pathways. As with phallotoxins, virotoxins are not considered to have significant toxic effects after oral exposure. At the molecular level, like phallotoxins, they interact with actin, stabilizing the bonds between actin monomers and preventing microfilaments depolymerization. However, the ultraviolet-spectra of interaction between actin and virotoxins is different from that of actin-phallotoxins, suggesting a different molecular interaction. Virotoxins have a more flexible structure when compared with phallotoxins and the presence of two additional hydroxyl groups may provide different reactivity. The intraperitoneal LD50 of virotoxins in mice ranges from 1.0 to 5.1 mg/kg and their main toxicological feature is hemorrhagic hepatic necrosis caused by an interaction of the virotoxins with outer surface of the hepatocyte through unknown mechanisms. At this point, the role of virotoxins in human toxicity remains unclear, although due to its poor oral absorption, little clinical importance is given to this class of toxins.

Luis Moroder is an Italian peptide chemist, who pioneered research on the interactions between peptide hormones and cell membrane-bound hormone receptors. He later expanded this research to other biological systems of medical relevance such as protein inhibitors, collagens, and synthetic proteins. A hallmark of his research is interdisciplinarity as reflected in his use and development of methods in organic chemistry, biophysics and molecular biology. He is a co-editor of the five-volume Houben-Weyl, Methods of Organic Chemistry, Synthesis of Peptides and Peptidomimetics. Since 2008 he is the editor-in-chief of the Journal of Peptide Science, the official journal of the European Peptide Society.














