
A lichen is a composite organism that arises from algae or cyanobacteria living among filaments of multiple fungi species in a mutualistic relationship. Lichens have properties different from those of their component organisms. They come in many colors, sizes, and forms and are sometimes plant-like, but lichens are not plants. They may have tiny, leafless branches (fruticose); flat leaf-like structures (foliose); crust-like, adhering tightly to a surface (substrate) like a thick coat of paint (crustose); a powder-like appearance (leprose); or other growth forms.

Lichen planus (LP) is a chronic inflammatory and immune-mediated disease that affects the skin, nails, hair, and mucous membranes. It is not an actual lichen, and is only named that because it looks like one. It is characterized by polygonal, flat-topped, violaceous papules and plaques with overlying, reticulated, fine white scale, commonly affecting dorsal hands, flexural wrists and forearms, trunk, anterior lower legs and oral mucosa. Although there is a broad clinical range of LP manifestations, the skin and oral cavity remain as the major sites of involvement. The cause is unknown, but it is thought to be the result of an autoimmune process with an unknown initial trigger. There is no cure, but many different medications and procedures have been used in efforts to control the symptoms.

Parmelia is a genus of medium to large foliose lichens. It has a global distribution, extending from the Arctic to the Antarctic continent but concentrated in temperate regions. There are about 40 species in Parmelia. In recent decades, the once large genus Parmelia has been divided into a number of smaller genera according to thallus morphology and phylogenetic relatedness.

Crotonic acid ((2E)-but-2-enoic acid) is a short-chain unsaturated carboxylic acid, described by the formula CH3CH=CHCO2H. It is called crotonic acid because it was erroneously thought to be a saponification product of croton oil. It crystallizes as colorless needles from hot water. The cis-isomer of crotonic acid is called isocrotonic acid. Crotonic acid is soluble in water and many organic solvents. Its odor is similar to butyric acid.

The Lecanoraceae are a family of lichenized fungi in the order Lecanorales. Species of this family have a widespread distribution.

The Baeomycetales are an order of mostly lichen-forming fungi in the subclass Ostropomycetidae, in the class Lecanoromycetes. It contains 8 families, 33 genera and about 170 species. As a result of molecular phylogenetics research published in the late 2010s, several orders were folded into the Baeomycetales, resulting in a substantial increase in the number of taxa.

Myelochroa is a genus of foliose lichens in the family Parmeliaceae. They are commonly known as axil-bristle lichens. It was created in 1987 to contain species formerly placed in genus Parmelina that had a yellow-orange medulla due to the presence of secalonic acids. Characteristics of the genus include tightly attached thalli with narrow lobes, cilia on the axils, and a rhizinate black lower surface. Chemical characteristics are the production of zeorin and related triterpenoids in the medulla. Myelochroa contains about 30 species, most of which grow on bark. The genus has centres of distribution in Asia and North America.
A spot test in lichenology is a spot analysis used to help identify lichens. It is performed by placing a drop of a chemical on different parts of the lichen and noting the colour change associated with application of the chemical. The tests are routinely encountered in dichotomous keys for lichen species, and they take advantage of the wide array of lichen products produced by lichens and their uniqueness among taxa. As such, spot tests reveal the presence or absence of chemicals in various parts of a lichen. They were first proposed by the botanist William Nylander in 1866.

Methylestradiol, sold under the brand names Ginecosid, Ginecoside, Mediol, and Renodiol, is an estrogen medication which is used in the treatment of menopausal symptoms. It is formulated in combination with normethandrone, a progestin and androgen/anabolic steroid medication. Methylestradiol is taken by mouth.

Prasterone enanthate, also known as dehydroepiandrosterone enanthate (DHEA-E) and sold in combination with estradiol valerate under the brand name Gynodian Depot among others, is a weak androgen, estrogen, and neurosteroid medication which is used as a component of menopausal hormone therapy to treat menopausal symptoms in women. It is available only as an injectable preparation in combination with estradiol valerate. The medication is given by injection into muscle typically once ever 4 weeks.

Physcia caesia, known colloquially as blue-gray rosette lichen and powder-back lichen, is a species of foliose lichenized fungus. First described by Georg Franz Hoffmann in 1784, it is common across much of Europe, North America and New Zealand, and more patchily distributed in South America, Asia, Australia and Antarctica. There are 2 subspecies: P. c. caesia and P. c. ventosa, as well as a number of distinct forms and varieties. Molecular studies suggest that the species as currently defined may be polyphyletic. It is typically pale gray shading to darker gray in the center, and grows in a small rosette, usually some 2–3 cm (0.79–1.18 in) across at maturity. It only rarely has apothecia, instead reproducing most often vegetatively via soredia, which are piled in round blue-gray mounds across the thallus's upper surface. It grows most often on rock—principally calcareous, but also basaltic and siliceous—and also occurs on bone, bark and soil. It is nitrophilic and is particularly common on substrates where birds perch.

Estradiol valerate/prasterone enanthate (EV/DHEA-E), sold under the brand name Gynodian Depot among others, is an injectable combination medication of estradiol valerate (EV), an estrogen, and prasterone enanthate (DHEA-E), an androgen, estrogen, and neurosteroid, which is used in menopausal hormone therapy for women. It is provided in the form of 1 mL ampoules containing 4 mg estradiol valerate and 200 mg prasterone enanthate in an oil solution and is administered by intramuscular injection once every 4 to 6 weeks. EV/DHEA-E reportedly has a duration of about 21 days.

Estradiol butyrylacetate/testosterone ketolaurate/reserpine (EBA/TKL/R), sold under the brand name Klimanosid R-Depot, is an injectable combination medication of estradiol butyrylacetate (EBA), an estrogen, testosterone ketolaurate, an androgen/anabolic steroid, and reserpine, an antipsychotic, which was previously used in menopausal hormone therapy for women, particularly in those with pronounced neurovegetative symptoms. It contains 2 mg EBA, 50 mg TKL, and 0.4 mg reserpine in oil solution in each 1 mL ampoule and is administered by intramuscular injection at regular intervals. The medication was marketed in 1957.

Psilolechia lucida is a species of saxicolous lichen in the family Psilolechiaceae. It is widely distributed through the world, where it grows on natural and artificial rocky substrates in the shade, often in sheltered underhangs. It forms a greenish crust on the surface of its substrate.
Thomas Hawkes Nash III is an American lichenologist. His research is about the biology and ecology of lichens, and the effects of air pollution on plants and lichens. He is known as an authority on the family Parmeliaceae. During his long career at the Arizona State University, he helped develop the lichen herbarium into a world-class collection with over 100,000 specimens representing more than 5000 species. In 2010, the year of his retirement, he was awarded the Acharius Medal for lifetime achievements in lichenology, and the following year had a Festschrift published in his honor.
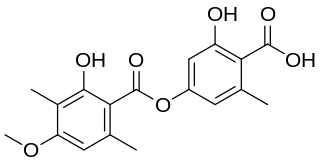
Ramalic acid is an organic compound with the molecular formula C18H18O7. Ramalic acid occurs as a secondary metabolite in some lichens like Ramalina pollinaria wherfrom ramalic acid has its name. Ramalic acid can be used as a dye.
A Phosphide chloride is a mixed anion compound containing both phosphide (P3−) and chloride (Cl−) ions.

Lepraria incana is a species of dust lichen in the family Stereocaulaceae. First described scientifically by Johann Jacob Dillenius in 1741, and then formally by Carl Linnaeus in 1753, it is the type species of the genus Lepraria. The thallus of this species is green to greyish-green, and powdery – as if made of tiny granules. These granules are soredia, which are asexual reproductive structures. Like most members of genus Lepraria, the lichen has few distinguishing features, lacking both a medulla and sexual reproductive structures (apothecia). Chemically, the lichen is characterised by the presence of the secondary chemicals known as divaricatic acid and zeorin.

Salazinic acid is a depsidone with a lactone ring. It is found in some lichens, and is especially prevalent in Parmotrema and Bulbothrix, where its presence or absence is often used to help classify species in those genera.

Thamnolic acid is a β-orcinol depside with the molecular formula C19H16O11. Thamnolic acid was first isolated from the lichen Thamnolia vermicularis, but it also occur in Cladonia spezies.

















