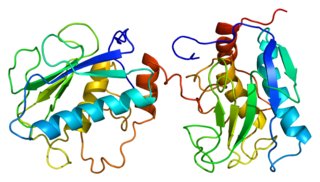An acetate is a salt formed by the combination of acetic acid with a base. "Acetate" also describes the conjugate base or ion typically found in aqueous solution and written with the chemical formula C
2H
3O−
2. The neutral molecules formed by the combination of the acetate ion and a positive ion are also commonly called "acetates". The simplest of these is hydrogen acetate with corresponding salts, esters, and the polyatomic anion CH
3CO−
2, or CH
3COO−
.
Saponification is a process of cleaving esters into carboxylate salts and alcohols by the action of aqueous alkali. Typically aqueous sodium hydroxide solutions are used. It is an important type of alkaline hydrolysis. When the carboxylate is long chain, its salt is called a soap. The saponification of ethyl acetate gives sodium acetate and ethanol:
Matrix metalloproteinases (MMPs), also known as matrix metallopeptidases or matrixins, are metalloproteinases that are calcium-dependent zinc-containing endopeptidases; other family members are adamalysins, serralysins, and astacins. The MMPs belong to a larger family of proteases known as the metzincin superfamily.

Zinc pyrithione is a coordination complex of zinc. It has fungistatic and bacteriostatic properties and is used in the treatment of seborrhoeic dermatitis and dandruff.

Valeric acid or pentanoic acid is a straight-chain alkyl carboxylic acid with the chemical formula CH3(CH2)3COOH. Like other low-molecular-weight carboxylic acids, it has an unpleasant odor. It is found in the perennial flowering plant Valeriana officinalis, from which it gets its name. Its primary use is in the synthesis of its esters. Salts and esters of valeric acid are known as valerates or pentanoates. Volatile esters of valeric acid tend to have pleasant odors and are used in perfumes and cosmetics. Several, including ethyl valerate and pentyl valerate are used as food additives because of their fruity flavors.
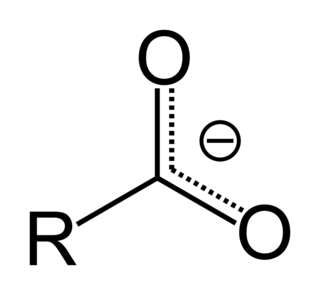
In organic chemistry, a carboxylate is the conjugate base of a carboxylic acid, RCOO−. It is an ion with negative charge.

The Knorr pyrrole synthesis is a widely used chemical reaction that synthesizes substituted pyrroles (3). The method involves the reaction of an α-amino-ketone (1) and a compound containing an electron-withdrawing group α to a carbonyl group (2).
Nucleophilic acyl substitution (SNAcyl) describes a class of substitution reactions involving nucleophiles and acyl compounds. In this type of reaction, a nucleophile – such as an alcohol, amine, or enolate – displaces the leaving group of an acyl derivative – such as an acid halide, anhydride, or ester. The resulting product is a carbonyl-containing compound in which the nucleophile has taken the place of the leaving group present in the original acyl derivative. Because acyl derivatives react with a wide variety of nucleophiles, and because the product can depend on the particular type of acyl derivative and nucleophile involved, nucleophilic acyl substitution reactions can be used to synthesize a variety of different products.
The Blaise ketone synthesis is the chemical reaction of acid chlorides with organozinc compounds to give ketones.
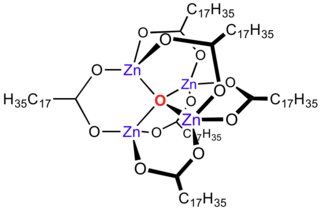
Zinc stearate is a "zinc soap" that is widely used industrially. In this context, soap is used in its formal sense, a metal salt of a fatty acid: in this case stearic acid. It is a white solid that repels water. It is insoluble in polar solvents such as alcohol and ether but soluble in aromatic hydrocarbons and chlorinated hydrocarbons when heated. It is the most powerful mold release agent among all metal soaps. It contains no electrolyte and has a hydrophobic effect. Its main application areas are the plastics and rubber industry, where it is used as a releasing agent and lubricant which can be easily incorporated.

Ricinoleic acid, formally called 12-hydroxy-9-cis-octadecenoic acid, is a fatty acid. It is an unsaturated omega-9 fatty acid and a hydroxy acid. It is a major component of the seed oil obtained from the seeds of castor plant, the plant that produces ricin. It is also found in the sclerotium of ergot. About 90% of the fatty acid content in castor oil is the ricinolein.

Carboxypeptidase A usually refers to the pancreatic exopeptidase that hydrolyzes peptide bonds of C-terminal residues with aromatic or aliphatic side-chains. Most scientists in the field now refer to this enzyme as CPA1, and to a related pancreatic carboxypeptidase as CPA2.
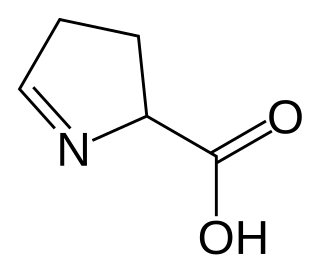
1-Pyrroline-5-carboxylic acid is a cyclic imino acid. Its conjugate base and anion is 1-pyrroline-5-carboxylate (P5C). In solution, P5C is in spontaneous equilibrium with glutamate-5-semialdhyde (GSA).
Stromelysin-1 also known as matrix metalloproteinase-3 (MMP-3) is an enzyme that in humans is encoded by the MMP3 gene. The MMP3 gene is part of a cluster of MMP genes which localize to chromosome 11q22.3. MMP-3 has an estimated molecular weight of 54 kDa.

In enzymology, an alanine racemase is an enzyme that catalyzes the chemical reaction
In enzymology, an aminoacylase (EC 3.5.1.14) is an enzyme that catalyzes the chemical reaction
Metal carbon dioxide complexes are coordination complexes that contain carbon dioxide ligands. Aside from the fundamental interest in the coordination chemistry of simple molecules, studies in this field are motivated by the possibility that transition metals might catalyze useful transformations of CO2. This research is relevant both to organic synthesis and to the production of "solar fuels" that would avoid the use of petroleum-based fuels.

Transition metal carboxylate complexes are coordination complexes with carboxylate (RCO2−) ligands. Reflecting the diversity of carboxylic acids, the inventory of metal carboxylates is large. Many are useful commercially, and many have attracted intense scholarly scrutiny. Carboxylates exhibit a variety of coordination modes, most common are κ1- (O-monodentate), κ2 (O,O-bidentate), and bridging.

Mugineic acid is the organic compound consisting of a azetidine group and three carboxylates. A colorless solid, it is a siderophore. More specifically, it is a phytosiderophore, i.e. a plant-produced siderophore. It functions as an iron accumulating agent for barley and other plants. Related phytosiderophores include nicotianamine and avenic acid.
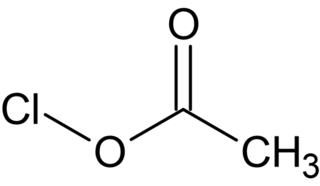
Acetyl hypochlorite, also known as chlorine acetate, is a chemical compound with the formula CH3COOCl. It is a photosensitive colorless liquid that is a short lived intermediate in the Hunsdiecker reaction.