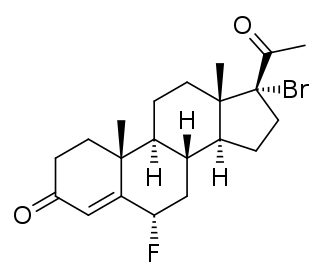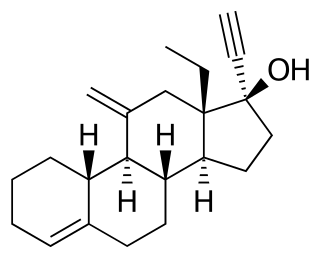
Desogestrel, sold under the brand names Cerazette and Mircette among many others, is a progestin medication which is used in birth control pills for women. It is also used in the treatment of menopausal symptoms in women. The medication is available and used alone or in combination with an estrogen. It is taken by mouth.
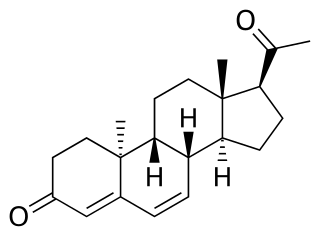
Dydrogesterone, sold under the brand name Duphaston among others, is a progestin medication which is used for a variety of indications, including threatened or recurrent miscarriage during pregnancy, dysfunctional bleeding, infertility due to luteal insufficiency, dysmenorrhea, endometriosis, secondary amenorrhea, irregular cycles, premenstrual syndrome, and as a component of menopausal hormone therapy. It is taken by mouth.

Ethisterone, also known as ethinyltestosterone, pregneninolone, and anhydrohydroxyprogesterone and formerly sold under the brand names Proluton C and Pranone among others, is a progestin medication which was used in the treatment of gynecological disorders but is now no longer available. It was used alone and was not formulated in combination with an estrogen. The medication is taken by mouth.

Hydroxyprogesterone caproate (OHPC), sold under the brand names Proluton and Makena among others, is a progestin medication which is used to prevent preterm birth in pregnant women with a history of the condition and to treat gynecological disorders. It is also used in combination with an estrogen as a form of long-lasting injectable birth control. It is not effective by mouth and must be given by injection into muscle, typically once per week.

Dimethisterone, formerly sold under the brand names Lutagan and Secrosteron among others, is a progestin medication which was used in birth control pills and in the treatment of gynecological disorders but is now no longer available. It was used both alone and in combination with an estrogen. It is taken by mouth.

Medrogestone, sold under the brand name Colprone among others, is a progestin medication which has been used in menopausal hormone therapy and in the treatment of gynecological disorders. It is available both alone and in combination with an estrogen. It is taken by mouth.

Promegestone, sold under the brand name Surgestone, is a progestin medication which is used in menopausal hormone therapy and in the treatment of gynecological disorders. It is taken by mouth.
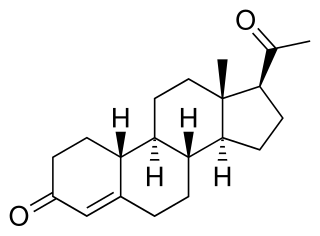
19-Norprogesterone, also known as 19-norpregn-4-ene-3,20-dione, is a steroidal progestin and close analogue of the sex hormone progesterone, lacking only the C19 methyl group of that molecule. It was first synthesized in 1944 in the form of a mixture that also included unnatural stereoisomers of progesterone, and this mixture was found to be at least equivalent to progesterone in terms of progestogenic activity. Subsequent investigations revealed that 17-isoprogesterone and 14-iso-17-isoprogesterone are devoid of progestogenic activity. 19-Norprogesterone was resynthesized in 1951 with an improved method, and was confirmed to be the component of the mixture synthesized in 1944 that was responsible for its progestogenic activity. In 1953, a paper was published showing that 19-norprogesterone possessed 4- to 8-fold the activity of progesterone in the Clauberg assay in rabbits, and at the time of this discovery, 19-norprogesterone was the most potent progestogen known.

Hydroxyprogesterone acetate (OHPA), sold under the brand name Prodox, is an orally active progestin related to hydroxyprogesterone caproate (OHPC) which has been used in clinical and veterinary medicine.

20α-Dihydroprogesterone (20α-DHP), also known as 20α-hydroxyprogesterone (20α-OHP), is a naturally occurring, endogenous progestogen. It is a metabolite of progesterone, converted by the 20α-hydroxysteroid dehydrogenases AKR1C1 and AKR1C3, and is still active as a progestogen. However, it is much less potent in comparison, with about one-fifth of the progestogenic activity of progesterone. It has been found to act as an aromatase inhibitor and to inhibit the production of estrogen in breast tissue in vitro.

Norgesterone, also known as norvinodrel or vinylestrenolone and sold under the brand name Vestalin, is a progestin medication which was formerly used in birth control pills for women but is now no longer marketed. It was used in combination with the estrogen ethinylestradiol. It is taken by mouth.

A progestogen ester is an ester of a progestogen or progestin. The prototypical progestogen is progesterone, an endogenous sex hormone. Esterification is frequently employed to improve the pharmacokinetics of steroids, including oral bioavailability, lipophilicity, and elimination half-life. In addition, with intramuscular injection, steroid esters are often absorbed more slowly into the body, allowing for less frequent administration. Many steroid esters function as prodrugs.

17α-Methylprogesterone (17α-MP), or 17α-methylpregn-4-ene-3,20-dione, is a steroidal progestin related to progesterone that was synthesized and characterized in 1949 but was never marketed. Along with ethisterone (1938) and 19-norprogesterone (1951), 17α-MP was one of the earliest derivatives of progesterone to be identified as possessing progestogenic activity. Similarly to progesterone and derivatives like 17α-hydroxyprogesterone and 19-norprogesterone, 17α-MP was found to possess poor oral bioavailability, but showed improved progestogenic activity relative to progesterone when administered via other routes. In addition to its activity as a progestogen, 17α-MP has also been found to possess some antiglucocorticoid activity.

11-Ketoprogesterone, or 11-oxoprogesterone, also known as pregn-4-ene-3,11,20-trione, is a pregnane steroid related to cortisone (11-keto-17α,21-dihydroxyprogesterone) that was formerly used in veterinary medicine in the treatment of bovine ketosis. It was synthesized in 1940. The steroid has profound effects on carbohydrate metabolism and possesses activities associated with adrenal cortex hormones like cortisone. However, it is non-toxic even in high dosages, suggesting that it lacks conventional glucocorticoid activity, and it does not possess mineralocorticoid activity, unlike other adrenocortical hormones. 11-Ketoprogesterone may act through membrane glucocorticoid receptors.

Progesterone 3-acetyl enol ether, also known as progesterone acetate, as well as 3-acetoxypregna-3,5-dien-20-one, is a progestin which was never marketed. It was reported to possess similar potency to progesterone and hydroxyprogesterone caproate in the rabbit endometrial carbonic anhydrase test, a bioassay of progestogenic activity. In addition, it was able to maintain pregnancy in animals. Progesterone 3-acetyl enol ether is closely related to quingestrone, which is also known as progesterone 3-cyclopentyl enol ether and was formerly marketed as an oral contraceptive.

DU-41164, also known as 1,2β-methylene-6-fluoro-17α-acetoxy-δ6-retroprogesterone, is a progestin which was developed by Philips-Duphar in the 1970s and was never marketed. It is a combined derivative of 17α-hydroxyprogesterone and retroprogesterone. The drug shows extremely high potency as a progestogen in animals; it was reported to possess 500 times the affinity of progesterone for the progesterone receptor expressed in rabbit uterus, and showed 600 times the progestogenic potency of subcutaneous progesterone when given orally in animals. The affinity of DU-41164 for the progesterone receptor was described in 1974 as "probably the highest reported for any steroid-receptor interaction". The drug showed no androgenic, anabolic, antiandrogenic, estrogenic, or corticosteroid activity in animals. Although highly potent in animals, DU-41164 produced little or no progestogenic effect at dosages of 50 and 200 µg/day in women, suggesting major species differences. A closely related compound, DU-41165, has been developed as a photoaffinity label for the progesterone receptor.
The pharmacology of progesterone, a progestogen medication and naturally occurring steroid hormone, concerns its pharmacodynamics, pharmacokinetics, and various routes of administration.

Methenmadinone acetate (MMA), also known as methylenedehydroacetoxyprogesterone (MDAP) and sold under the brand names Superlutin and Antigest, is a progestin medication which was developed in Czechoslovakia in the 1960s. It is the C17α acetate ester of methenmadinone.