A progestogen, also referred to as a progestagen, gestagen, or gestogen, is a type of medication which produces effects similar to those of the natural female sex hormone progesterone in the body. A progestin is a synthetic progestogen. Progestogens are used most commonly in hormonal birth control and menopausal hormone therapy. They can also be used in the treatment of gynecological conditions, to support fertility and pregnancy, to lower sex hormone levels for various purposes, and for other indications. Progestogens are used alone or in combination with estrogens. They are available in a wide variety of formulations and for use by many different routes of administration. Examples of progestogens include natural or bioidentical progesterone as well as progestins such as medroxyprogesterone acetate and norethisterone.

Norgestimate, sold under the brand names Ortho Tri-Cyclen and Previfem among others, is a progestin medication which is used in birth control pills for women and in menopausal hormone therapy. The medication is available in combination with an estrogen and is not available alone. It is taken by mouth.

Chlormadinone acetate (CMA), sold under the brand names Belara, Gynorelle, Lutéran, and Prostal among others, is a progestin and antiandrogen medication which is used in birth control pills to prevent pregnancy, as a component of menopausal hormone therapy, in the treatment of gynecological disorders, and in the treatment of androgen-dependent conditions like enlarged prostate and prostate cancer in men and acne and hirsutism in women. It is available both at a low dose in combination with an estrogen in birth control pills and, in a few countries like France and Japan, at low, moderate, and high doses alone for various indications. It is taken by mouth.

Melengestrol acetate (MLGA), sold under the brand names Heifermax and MGA among others, is a progestin medication which is used in animal reproduction. It is not approved for use in humans, and is instead used as an implantable contraceptive for captive animals in zoos and other refuges, and is also used as a feed additive to promote growth in cattle, a purpose it is licensed for in the United States and Canada.

Allylestrenol, sold under the brand names Gestanin and Turinal among others, is a progestin medication which is used to treat recurrent and threatened miscarriage and to prevent premature labor in pregnant women. However, except in the case of proven progesterone deficiency, its use for such purposes is no longer recommended. It is also used in Japan to treat benign prostatic hyperplasia (BPH) in men. The medication is used alone and is not formulated in combination with an estrogen. It is taken by mouth.

Nomegestrol acetate (NOMAC), sold under the brand names Lutenyl and Zoely among others, is a progestin medication which is used in birth control pills, menopausal hormone therapy, and for the treatment of gynecological disorders. It is available both alone and in combination with an estrogen. NOMAC is taken by mouth. A birth control implant for placement under the skin was also developed but ultimately was not marketed.
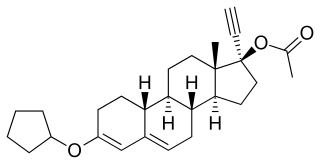
Quingestanol acetate, sold under the brand names Demovis and Pilomin among others, is a progestin medication which was used in birth control pills but is no longer marketed. It is taken by mouth.

Delmadinone acetate (DMA), sold under the brand name Tardak among others, is a progestin and antiandrogen which is used in veterinary medicine to treat androgen-dependent conditions such as benign prostatic hyperplasia. It must be used with care as it has the potential to cause adrenal insufficiency via inhibition of adrenocorticotropic hormone (ACTH) secretion from the pituitary gland. DMA is the C17α acetate ester of delmadinone, which, in contrast to DMA, was never marketed for medical use.

Segesterone acetate (SGA), sold under the brand names Nestorone, Elcometrine, and Annovera, is a progestin medication which is used in birth control and in the treatment of endometriosis in the United States, Brazil, and other South American countries. It is available both alone and in combination with an estrogen. It is not effective by mouth and must be given by other routes, most typically as a vaginal ring or implant that is placed into fat.
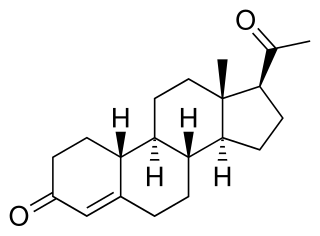
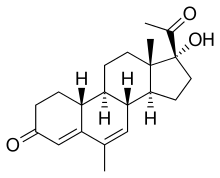
19-Norprogesterone, also known as 19-norpregn-4-ene-3,20-dione, is a steroidal progestin and close analogue of the sex hormone progesterone, lacking only the C19 methyl group of that molecule. It was first synthesized in 1944 in the form of a mixture that also included unnatural stereoisomers of progesterone, and this mixture was found to be at least equivalent to progesterone in terms of progestogenic activity. Subsequent investigations revealed that 17-isoprogesterone and 14-iso-17-isoprogesterone are devoid of progestogenic activity. 19-Norprogesterone was resynthesized in 1951 with an improved method, and was confirmed to be the component of the mixture synthesized in 1944 that was responsible for its progestogenic activity. In 1953, a paper was published showing that 19-norprogesterone possessed 4- to 8-fold the activity of progesterone in the Clauberg assay in rabbits, and at the time of this discovery, 19-norprogesterone was the most potent progestogen known.

Hydroxyprogesterone acetate (OHPA), sold under the brand name Prodox, is an orally active progestin related to hydroxyprogesterone caproate (OHPC) which has been used in clinical and veterinary medicine. It has reportedly also been used in birth control pills.
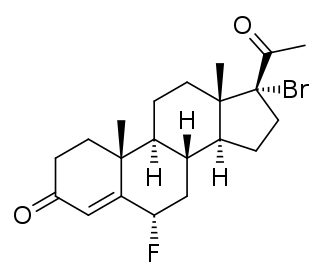
Haloprogesterone, sold under the brand name Prohalone, is a progestin medication which was previously marketed by Ayerst but is now no longer available.
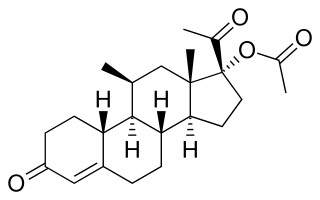
Norgestomet, or norgestamet, sold under the brand name Syncro-Mate B and Crestar, is a progestin medication which is used in veterinary medicine to control estrus and ovulation in cattle.

Anagestone acetate, sold under the brand names Anatropin and Neo-Novum, is a progestin medication which was withdrawn from medical use due to carcinogenicity observed in animal studies.
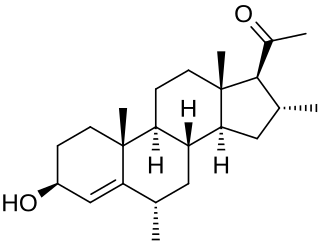
Dimepregnen, or 6α,16α-dimethylpregn-4-en-3β-ol-20-one, is a pregnene steroid described as an antiestrogen that was synthesized in 1968 and was never marketed. It is similar in structure to the progestins and progesterone derivatives melengestrol and anagestone.
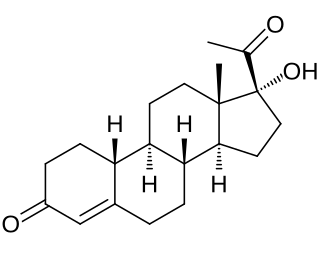
Gestronol (BAN), also known as gestonorone, as well as 17α-hydroxy-19-norprogesterone or 17α-hydroxy-19-norpregn-4-ene-3,20-dione, is a progestin of the 19-norprogesterone and 17α-hydroxyprogesterone groups which was never marketed. The C17α caproate ester of gestronol, gestonorone caproate, in contrast, has been marketed.

A progestogen ester is an ester of a progestogen or progestin. The prototypical progestogen is progesterone, an endogenous sex hormone. Esterification is frequently employed to improve the pharmacokinetics of steroids, including oral bioavailability, lipophilicity, and elimination half-life. In addition, with intramuscular injection, steroid esters are often absorbed more slowly into the body, allowing for less frequent administration. Many steroid esters function as prodrugs.
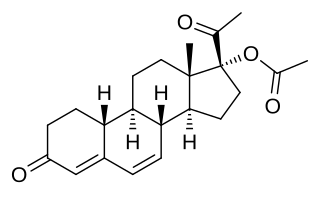
Gestadienol acetate an orally active progestin which was described in the literature in 1967 and was never marketed. It has no androgenic or estrogenic effects. The effects of gestadienol acetate on the endometrium and its general pharmacology were studied in a clinical trial in women. It has also been studied in a clinical trial for benign prostatic hyperplasia in men, but was ineffective.

17α-Allyl-19-nortestosterone, also known as 3-ketoallylestrenol or as 17α-allylestr-4-en-17β-ol-3-one, is a progestin which was never marketed. It is a combined derivative of the anabolic–androgenic steroid and progestogen nandrolone (19-nortestosterone) and the antiandrogen allyltestosterone (17α-allyltestosterone). The drug is a major active metabolite of allylestrenol, which is thought to be a prodrug of 17α-allyl-19-nortestosterone.