
Antiandrogens, also known as androgen antagonists or testosterone blockers, are a class of drugs that prevent androgens like testosterone and dihydrotestosterone (DHT) from mediating their biological effects in the body. They act by blocking the androgen receptor (AR) and/or inhibiting or suppressing androgen production. They can be thought of as the functional opposites of AR agonists, for instance androgens and anabolic steroids (AAS) like testosterone, DHT, and nandrolone and selective androgen receptor modulators (SARMs) like enobosarm. Antiandrogens are one of three types of sex hormone antagonists, the others being antiestrogens and antiprogestogens.

Bicalutamide, sold under the brand name Casodex among others, is an antiandrogen medication that is primarily used to treat prostate cancer. It is typically used together with a gonadotropin-releasing hormone (GnRH) analogue or surgical removal of the testicles to treat advanced prostate cancer. To a lesser extent, it is used for early prostate cancer at a higher dosage as a monotherapy without castration. Bicalutamide is also used to treat excessive hair growth and scalp hair loss in women, as a component of feminizing hormone therapy for transgender women, to treat early puberty in boys, and to prevent overly long-lasting erections in men. It is taken by mouth.

Nilutamide, sold under the brand names Nilandron and Anandron, is a nonsteroidal antiandrogen (NSAA) which is used in the treatment of prostate cancer. It has also been studied as a component of feminizing hormone therapy for transgender women and to treat acne and seborrhea in women. It is taken by mouth.

Cytochrome P450 17A1 is an enzyme of the hydroxylase type that in humans is encoded by the CYP17A1 gene on chromosome 10. It is ubiquitously expressed in many tissues and cell types, including the zona reticularis and zona fasciculata of the adrenal cortex as well as gonadal tissues. It has both 17α-hydroxylase and 17,20-lyase activities, and is a key enzyme in the steroidogenic pathway that produces progestins, mineralocorticoids, glucocorticoids, androgens, and estrogens. More specifically, the enzyme acts upon pregnenolone and progesterone to add a hydroxyl (-OH) group at carbon 17 position (C17) of the steroid D ring, or acts upon 17α-hydroxyprogesterone and 17α-hydroxypregnenolone to split the side-chain off the steroid nucleus.

Abiraterone acetate, sold under the brand name Zytiga among others, is a medication used to treat prostate cancer. Specifically it is used together with a corticosteroid for metastatic castration-resistant prostate cancer (mCRPC) and metastatic high-risk castration-sensitive prostate cancer (mCSPC). It should either be used following removal of the testicles or along with a gonadotropin-releasing hormone (GnRH) analog. It is taken by mouth.

Enzalutamide, sold under the brand name Xtandi, is a nonsteroidal antiandrogen (NSAA) medication which is used in the treatment of prostate cancer. It is indicated for use in conjunction with castration in the treatment of metastatic castration-resistant prostate cancer (mCRPC), nonmetastatic castration-resistant prostate cancer, and metastatic castration-sensitive prostate cancer (mCSPC). It is taken by mouth.
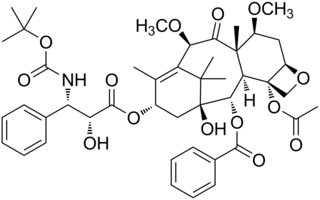
Cabazitaxel, sold under the brand name Jevtana, is a semi-synthetic derivative of a natural taxoid. It is a microtubule inhibitor, and the fourth taxane to be approved as a cancer therapy.

Galeterone is a steroidal antiandrogen which was under development by Tokai Pharmaceuticals for the treatment of prostate cancer. It possesses a unique triple mechanism of action, acting as an androgen receptor antagonist, androgen receptor down regulator, and CYP17A1 inhibitor, the latter of which prevents the biosynthesis of androgens. As a CYP17A1 inhibitor, galeterone shows selectivity for 17,20-lyase over 17α-hydroxylase.
EPI-001 is the first inhibitor of the androgen receptor amino-terminal domain. The single stereoisomer of EPI-001, EPI-002, is a first-in-class drug that the USAN council assigned a new stem class "-aniten" and the generic name "ralaniten". This distinguishes the anitens novel molecular mechanism from anti androgens that bind the C-terminus ligand-binding domain and have the stem class "lutamide". EPI-001 and its stereoisomers and analogues were discovered by Marianne Sadar and Raymond Andersen, who co-founded the pharmaceutical company ESSA Pharma Inc for the clinical development of anitens for the treatment of castration-resistant prostate cancer (CRPC).

A nonsteroidal antiandrogen (NSAA) is an antiandrogen with a nonsteroidal chemical structure. They are typically selective and full or silent antagonists of the androgen receptor (AR) and act by directly blocking the effects of androgens like testosterone and dihydrotestosterone (DHT). NSAAs are used in the treatment of androgen-dependent conditions in men and women. They are the converse of steroidal antiandrogens (SAAs), which are antiandrogens that are steroids and are structurally related to testosterone.

Seviteronel is an experimental cancer medication which is under development by Viamet Pharmaceuticals and Innocrin Pharmaceuticals for the treatment of prostate cancer and breast cancer. It is a nonsteroidal CYP17A1 inhibitor and works by inhibiting the production of androgens and estrogens in the body. As of July 2017, seviteronel is in phase II clinical trials for both prostate cancer and breast cancer. In January 2016, it was designated fast-track status by the United States Food and Drug Administration for prostate cancer. In April 2017, seviteronel received fast-track designation for breast cancer as well.
Darolutamide, sold under the brand name Nubeqa, is an antiandrogen medication which is used in the treatment of non-metastatic castration-resistant prostate cancer in men. It is specifically approved to treat non-metastatic castration-resistant prostate cancer (nmCRPC) in conjunction with surgical or medical castration. The medication is taken by mouth twice per day with food.

Apalutamide, sold under the brand name Erleada among others, is a nonsteroidal antiandrogen (NSAA) medication which is used in the treatment of prostate cancer. It is specifically indicated for use in conjunction with castration in the treatment of non-metastatic castration-resistant prostate cancer (NM-CRPC). It is taken by mouth.

Ralaniten acetate is a first-in-class antiandrogen that targets the N-terminal domain (NTD) of the androgen receptor (AR) developed by ESSA Pharmaceuticals and was under investigation for the treatment of prostate cancer. This mechanism of action is believed to allow the drug to block signaling from the AR and its splice variants. EPI-506 is a derivative of bisphenol A and a prodrug of ralaniten (EPI-002), one of the four stereoisomers of EPI-001, and was developed as a successor of EPI-001. The drug reached phase I/II prior to the discontinuation of its development. It showed signs of efficacy in the form of prostatic specific antigen (PSA) decreases (4–29%) predominantly at higher doses (≥1,280 mg) in some patients but also caused side effects and was discontinued by its developer in favor of next-generation AR NTD inhibitors with improved potency and tolerability.

Ketodarolutamide is a nonsteroidal antiandrogen (NSAA) and the major active metabolite of darolutamide, an NSAA which is used in the treatment of prostate cancer in men. Similarly to its parent compound, darolutamide acts as a highly selective, high-affinity, competitive silent antagonist of the androgen receptor (AR). Both agents show much higher affinity and more potent inhibition of the AR relative to the other NSAAs enzalutamide and apalutamide, although they also possess much shorter and comparatively less favorable elimination half-lives. They have also been found not to activate certain mutant AR variants that enzalutamide and apalutamide do activate. Both darolutamide and ketodarolutamide show limited central nervous system distribution, indicating peripheral selectivity, and little or no inhibition or induction of cytochrome P450 enzymes such as CYP3A4, unlike enzalutamide and apalutamide.
The side effects of bicalutamide, a nonsteroidal antiandrogen (NSAA), including its frequent and rare side effects, have been well-studied and characterized. The most common side effects of bicalutamide monotherapy in men include breast tenderness, gynecomastia, feminization, demasculinization, and hot flashes. Less common side effects of bicalutamide monotherapy in men include sexual dysfunction, depression, fatigue, weakness, and anemia. Bicalutamide is well tolerated and has few side effects in women. General side effects of bicalutamide that may occur in either sex include diarrhea, constipation, abdominal pain, nausea, dry skin, itching, and rash.
Comparison of the nonsteroidal antiandrogen (NSAA) bicalutamide with other antiandrogens reveals differences between the medications in terms of efficacy, tolerability, safety, and other parameters. Relative to the other first-generation NSAAs, flutamide and nilutamide, bicalutamide shows improved potency, efficacy, tolerability, and safety, and has largely replaced these medications in clinical practice. Compared to the second-generation NSAAs, enzalutamide and apalutamide, bicalutamide has inferior potency and efficacy but similar tolerability and safety and a lower propensity for drug interactions.

The pharmacology of bicalutamide, a nonsteroidal antiandrogen (NSAA), has been well-characterized. In terms of pharmacodynamics, bicalutamide acts as a selective antagonist of the androgen receptor (AR), the biological target of androgens like testosterone and dihydrotestosterone (DHT). It has no capacity to activate the AR. It does not decrease androgen levels and has no other important hormonal activity. The medication has progonadotropic effects due to its AR antagonist activity and can increase androgen, estrogen, and neurosteroid production and levels. This results in a variety of differences of bicalutamide monotherapy compared to surgical and medical castration, such as indirect estrogenic effects and associated benefits like preservation of sexual function and drawbacks like gynecomastia. Bicalutamide can paradoxically stimulate late-stage prostate cancer due to accumulated mutations in the cancer. When used as a monotherapy, bicalutamide can induce breast development in males due to its estrogenic effects. Unlike other kinds of antiandrogens, it may have less adverse effect on the testes and fertility.

RU-59063 is a nonsteroidal androgen or selective androgen receptor modulator (SARM) which was first described in 1994 and was never marketed. It was originally thought to be a potent antiandrogen, but subsequent research found that it actually possesses dose-dependent androgenic activity, albeit with lower efficacy than dihydrotestosterone (DHT). The drug is an N-substituted arylthiohydantoin and was derived from the first-generation nonsteroidal antiandrogen (NSAA) nilutamide. The second-generation NSAAs enzalutamide, RD-162, and apalutamide were derived from RU-59063.
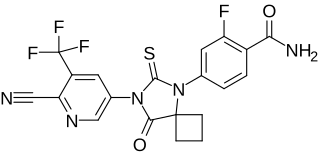
N-Desmethylapalutamide is a nonsteroidal antiandrogen (NSAA) and the major active metabolite of apalutamide, an NSAA which is used as a hormonal antineoplastic agent in the treatment of metastatic prostate cancer. It has similar activity to that of apalutamide and, with apalutamide therapy, circulates at similar concentrations to those of apalutamide at steady state. N-Desmethylapalutamide is formed from apalutamide in the liver by the cytochrome P450 enzymes CYP2C8 and CYP3A4.













